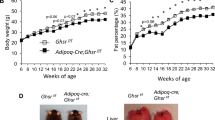
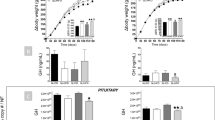
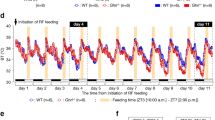
Abstract
BACKGROUND: Ghrelin, an endogenous ligand for growth hormone secretagogue receptor (GHS-R), is known to increase food intake in lean humans and rodents. In addition, ghrelin levels are increased by fasting in lean rodents and are elevated before meals in humans, suggesting an important role for ghrelin in meal initiation. However, in obese human, circulating ghrelin levels were found to be significantly reduced as compared to lean individuals.
OBJECTIVES: To evaluate whether circulating ghrelin levels, as well as ghrelin sensitivity, are decreased in obese individuals in order to limit its effect on food intake.
DESIGN: Lean C57BL/6J mice fed a chow, a low- (LFD) or a high-fat diet (HFD) were used to determine ghrelin regulation and secretion as well as ghrelin sensitivity.
MEASUREMENTS: Plasma ghrelin levels were measured in low- and high-fat fed mice. Ghrelin-induced food intake was measured in chow, low- and high-fat fed mice.
RESULTS: We measured ghrelin levels in lean and diet-induced obese mice, fed on an LFD or an HFD, respectively. We observed that not only ghrelin secretion was reduced in obese mice but its diurnal regulation was also lost. In addition, we failed to observe any change in ghrelin secretion upon fasting and refeeding. Moreover, we observed that the sensitivity to the orexigenic effects of exogenous ghrelin was reduced in obese mice when compared to lean mice fed a chow or a LFD. The insensitivity of obese mice to ghrelin was improved upon weigh loss.
CONCLUSION: Altogether, these results indicate that ghrelin secretion and regulation is impaired in dietary-induced obesity in mice and suggest that ghrelin inhibition could prevent weight regain after weight loss.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M . The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002; 87: 2988.
Hosoda H, Kojima M, Matsuo H, Kangawa K . Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 2000; 279: 909–913.
Yoshihara F, Kojima M, Hosoda H, Nakazato M, Kangawa K . Ghrelin: a novel peptide for growth hormone release and feeding regulation. Curr Opin Clin Nutr Metab Care 2002; 5: 391–395.
Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M . The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002; 123: 1120–1128.
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S . A role for ghrelin in the central regulation of feeding. Nature 2001; 409: 194–198.
Bagnasco M, Tulipano G, Melis MR, Argiolas A, Cocchi D, Muller EE . Endogenous ghrelin is an orexigenic peptide acting in the arcuate nucleus in response to fasting. Regul Peptides 2003; 111: 161–167.
Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K . Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 2001; 50: 227–232.
Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M . Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001; 120: 337–345.
Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S . Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun 2001; 280: 904–907.
Casanueva FF, Dieguez C . Ghrelin: the link connecting growth with metabolism and energy homeostasis. Rev Endocr Metab Disord 2002; 3: 325–338.
Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR . The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000; 141: 4325–4328.
Tschop M, Smiley DL, Heiman ML . Ghrelin induces adiposity in rodents. Nature 2000; 407: 908–913.
Murakami N, Hauashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K . Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol 2002; 174: 283–288.
Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR . Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86: 5992.
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS . A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001; 50: 1714–1719.
Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR . Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001; 50: 2540–2547.
Ariyasu H, Takaya K, Hosoda H, Iwakura H, Ebihara K, Mori K, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K . Delayed short-term secretory regulation of ghrelin in obese animals: evidenced by a specific RIA for the active form of ghrelin. Endocrinology 2002; 143: 3341–3350.
Rigamonti AE, Pincelli AI, Corra B, Viarengo R, Bonomo SM, Galimberti D, Scacchi M, Scarpini E, Cavagnini F, Muller EE . Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. J Endocrinol 2002; 175: R1–R5.
Beck B, Musse N, Stricker-Krongrad A . Ghrelin, macronutrient intake and dietary preferences in long-evans rats. Biochem Biophys Res Commun 2002; 292: 1031–1035.
Ikezaki A, Hosoda H, Ito K, Iwama S, Miura N, Matsuoka H, Kondo C, Kojima M, Kangawa K, Sugihara S . Fasting plasma ghrelin levels are negatively correlated with insulin resistance and PAI-1, but not with leptin, in obese children and adolescents. Diabetes 2002; 51: 3408–3411.
Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, Jorgensen JO . Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxford) 2002; 56: 203–206.
Bush EN, Droz B, Shaprio R, Knourek-Segel VE, Fey T, Bruen ME, Fagerland J, Jacobson PB, Collins C, Kaszubska W, Cummings DE . Effects of low-calorie or low-fat diet-induced weight loss on plasma ghrelin levels. Endocrinology 2003, Endo 2003 Endocrine society's 85th Annual Meeting, p. 398.
Lin S, Thomas TC, Storlien LH, Huang XF . Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord 2000; 24: 639–646.
Prpic V, Watson PM, Frampton IC, Sabol MA, Jezek GE, Gettys TW . Differential mechanisms and development of leptin resistance in A/J versus C57BL/6J mice during diet-induced obesity. Endocrinology 2003; 144: 1155–1163.
Possidente B, Birnbaum S . Circadian rhythms for food and water consumption in the mouse, Mus musculus. Physiol Behav 1979; 22: 657–660.
Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ . Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002; 346: 1623–1630.
Asakawa A, Inui A, Kaga T, Katsuura G, Fujjimiya M, Fujino MA, Kasuga M . Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 2003; 52: 947–952.
Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP, Sybertz EJ, Strader CD, Davis Jr. HR . Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 1997; 99: 385–390.
Lin L, Martin R, Schaffhauser AO, York DA . Acute changes in the response to peripheral leptin with alteration in the diet composition. Am J Physiol Regul Integr Comp Physiol 2001; 280: R504–R509.
Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL . The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes 2002; 51: 3412–3419.
Traebert M, Riediger T, Whitebread S, Scharrer E, Schmid HA . Ghrelin acts on leptin-responsive neurones in the rat arcuate nucleus. J Neuroendocrinol 2002; 14: 580–586.
Cowley MA, Cone RD, Enriori P, Louiselle I, Williams SM, Evans AE . Electrophysiological actions of peripheral hormones on melanocortin neurons. Ann NY Acad Sci 2003; 994: 175–186.
Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, Shioda S, Matsukura S, Kangawa K, Nakazato M . Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology 2003; 144: 1506–1512.
Wang L, Saint-Pierre DH, Tache Y . Peripheral ghrelin selectively increases Fos expression in neuropeptide Y—synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 2002; 325: 47–51.
Stricker-Krongrad A, Richy S, Beck B . Orexins/hypocretins in the ob/ob mouse: hypothalamic gene expression, peptide content and metabolic effects. Regul Peptides 2002; 104: 11–20.
Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS . Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 2000; 279: E838–E845.
Smith FJ, Campfield LA, Moschera JA, Bailon PS, Burn P . Brain administration of OB protein (leptin) inhibits neuropeptide-Y-induced feeding in ob/ob mice. Regul Peptides 1998; 75–76: 433–439.
Lawrence CB, Snape AC, Baudoin FM, Luckman SM . Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 2002; 143: 155–162.
Melis MR, Mascia MS, Succu S, Torsello A, Muller EE, Denghenghi R, Argiolas A . Ghrelin injected into the paraventricular nucleus of the hypothalamus of male rats induces feeding but not penile erection. Neurosci Lett 2002; 329: 339–343.
Acknowledgements
We thank Biomedical Research Model (BRM), the Animal Resource Group (ARG) and Margarita Camara as well as Sandhya Punreddy for their technical assistance. We thank Paul Richardson from Abbott Laboratories (Abbott Park, IL, USA) for ghrelin peptide supply. Finally, we address a special thank to Eugene Bush and his team at Abbott Laboratories for their effort in developing the high-fat to low-fat switch model.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perreault, M., Istrate, N., Wang, L. et al. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int J Obes 28, 879–885 (2004). https://doi.org/10.1038/sj.ijo.0802640
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802640
Keywords
This article is cited by
-
Sex-specific differences in metabolic hormone and adipose tissue dynamics induced by moderate low-carbohydrate and ketogenic diet
Scientific Reports (2023)
-
Corticotropin-releasing factor overexpression in mice abrogates sex differences in body weight, visceral fat, and food intake response to a fast and alters levels of feeding regulatory hormones
Biology of Sex Differences (2017)
-
The cellular and molecular bases of leptin and ghrelin resistance in obesity
Nature Reviews Endocrinology (2017)
-
Neonatal events, such as androgenization and postnatal overfeeding, modify the response to ghrelin
Scientific Reports (2014)
-
Characterization of Ghrelin in Pedigreed Baboons: Evidence for Heritability and Pleiotropy
Obesity (2008)