Abstract
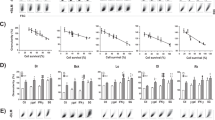
This report examines a major barrier to suicide gene therapy in cancer and other diseases: namely, bystander cell killing. Existing vectors for in vivo gene delivery are inefficient and often transduce or transfect less than 1% of target cells. The E. coli PNP gene brings about cellular necrosis under conditions when 1 in 100 to 1 in 1000 cells express the gene product in vitro. In vivo bystander killing at or near this magnitude has not been reported previously. In the present experiments, transfection of cells with the E. coli PNP gene controlled by a SV40 promoter resulted in 30 nmol 6-methyl purine deoxyriboside (MeP-dR) converted per milligram tumor cell extract per hour (or conversion units (CU)). This level of expression led to elimination of entire populations of tumor cells in vitro after treatment with MeP-dR. Much earlier killing was observed using a tat transactivated E. coli PNP vector (approximately seven-fold higher activity, 230 CU). In vivo effects on tumor growth were next examined. Human ovarian tumors transfected with E. coli PNP were excised 5 days after i.p. implantation from the peritoneal cavities of mice in order to determine both E. coli PNP enzymatic activity and the fraction of cells expressing the gene. PNP activity at 5 days after gene transfer was approximately 170 CU and was expressed in approximately 0.1% of the tumor cells as judged by in situ hybridization. The expression of E. coli PNP at this level produced a 30% increase in life span (P < 0.001) and 49% reduction in tumor size (P < 0.005) after MeP-dR treatment, as compared with control tumors. Our observations lead to the conclusion that pronounced bystander killing by E. coli PNP is conferred in vivo, and that vectors capable of transgene expression in as few as one in 1000 cells can produce substantial antitumor effects if expression on a per cell basis is very high.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sorscher EJ et al. Tumor cell bystander killing in colonic carcinoma utilizing the E. coli Deo D gene to generate toxic purines Gene Therapy 1994 1: 233–238
Hughes BW et al. Bystander killing of melanoma cells using the human tyrosinase promoter to express the Escherichia coli purine nucleoside phosphorylase gene Cancer Res 1995 55: 3339–3345
Da Costa LT et al. Converting cancer genes into killer genes Proc Natl Acad Sci USA 1996 93: 4192–4196
Parker WB et al. In vivo gene therapy of cancer with E. coli purine nucleoside phosphorylase Hum Gene Ther 1997 8: 1637–1644
Hughes BW et al. Cell to cell contact is not required for bystander cell killing by Escherichia coli purine nucleoside phosphorylase J Biol Chem 1998 273: 2322–2328
Parker WB et al. Metabolism and metabolic action of 6-methylpurine and 2-fluoroadenine in human cells Biochem Pharm 1998 55: 1673–1681
Lockett LJ, Molloy PL, Russell PJ, Both GW . Relative efficiency of tumor cell killing in vitro by two enzyme-prodrug systems delivered by identical adenovirus vectors Clin Cancer Res 1997 3: 2075–2080
Martiniello-Wilks R et al. In vivo gene therapy for prostate cancer: preclinical evaluation of two different enzyme-directed prodrug therapy systems delivered by identical adenovirus vectors Hum Gene Ther 1998 9: 1617–1626
Puhlmann M et al. Thymidine kinase-deleted vaccinia virus expressing purine nucleoside phosphorylase as a vector for tumor-directed gene therapy Hum Gene Ther 1999 10: 649–657
Jensen KF, Nygaard P . Purine nucleoside phosphorylase from Escherichia coli and Salmonella typhimurium. Purification and some properties Eur J Biochem 1975 51: 253–265
Mao C et al. The crystal structure of Escherichia coli purine nucleoside phosphorylase: a comparison with the human enzyme reveals a conserved topology Structure 1997 5: 1373–1383
Nestler U et al. Foamy virus vectors for suicide gene therapy Gene Therapy 1997 4: 1270–1277
Niculescu-Duvaz I, Spooner R, Marais R, Springer CJ . Gene-directed enzyme prodrug therapy Bioconjug Chem 1998 9: 4–22
Hoganson DK, Batra RK, Olsen JC, Boucher RC . Comparison of the effects of three different toxin genes and their levels of expression on cell growth and bystander effect in lung adenocarcinoma Cancer Res 1996 56: 1315–1323
Beck C et al. The thymidine kinase/ganciclovir-mediated ‘suicide’ effect is variable in different tumor cells Hum Gene Ther 1995 6: 1525–1530
Dilber MS et al. Gap junctions promote the bystander effect of herpes simplex virus thymidine kinase in vivo Cancer Res 1997 57: 1523–1528
Elshami AA et al. Gap junctions play a role in the ‘bystander effect’ of the herpes simplex virus thymidine kinase/ganciclovir system in vitro Gene Therapy 1996 3: 85–92
Fick J et al. The extent of heterocellular communication mediated by gap junctions is predictive of bystander tumor cytotoxicity in vitro Proc Natl Acad Sci USA 1995 92: 11071–11055
Imaizumi K et al. Bystander tumoricidal effect gap junctional communication in lung cancer cell lines Am J Respir Cell Mol Biol 1998 18: 205–212
Freeman SM et al. The ‘bystander effect’: tumor regression when a fraction of the tumor mass is genetically modified Cancer Res 1993 53: 5274–5283
Sacco MG et al. Partial regression, yet incomplete eradication of mammary tumors in transgenic mice by retrovirally mediated HSVtk transfer in vivo Gene Therapy 1996 3: 1151–1156.
Marini FCr, Nelson JA, Lapeyre JN . Assessment of bystander effect potency produced by intratumoral implantation of HSVtk-expressing cells using surrogate marker secretion to monitor tumor growth kinetics Gene Therapy 1995 2: 655–659
Vile RG et al. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component Cancer Res 1994 54: 6228–6234
Caruso M et al. Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma Proc Natl Acad Sci USA 1996 93: 11302–11306
Tapscott SJ et al. Gene therapy of rat 9L gliosarcoma tumors by transduction with selectable genes does not require drug selection Proc Natl Acad Sci USA 1994 91: 8185–8189
Huber BE et al. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase Proc Natl Acad Sci USA 1994 91: 8302–8306
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gadi, V., Alexander, S., Kudlow, J. et al. In vivo sensitization of ovarian tumors to chemotherapy by expression of E. coli purine nucleoside phosphorylase in a small fraction of cells. Gene Ther 7, 1738–1743 (2000). https://doi.org/10.1038/sj.gt.3301286
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3301286
Keywords
This article is cited by
-
Suicide gene strategies applied in ovarian cancer studies
Cancer Gene Therapy (2023)
-
Targeting purine metabolism in ovarian cancer
Journal of Ovarian Research (2022)
-
Pseudomonas aeruginosa NfsB and nitro-CBI-DEI – a promising enzyme/prodrug combination for gene directed enzyme prodrug therapy
Molecular Cancer (2013)
-
Tumor-targeting Salmonella typhimurium, a natural tool for activation of prodrug 6MePdR and their combination therapy in murine melanoma model
Applied Microbiology and Biotechnology (2013)
-
Purine Nucleoside Phosphorylase mediated molecular chemotherapy and conventional chemotherapy: A tangible union against chemoresistant cancer
BMC Cancer (2011)