Abstract
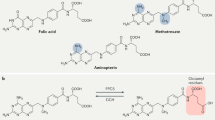
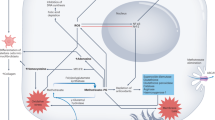
Methotrexate remains a cornerstone in the treatment of rheumatoid arthritis and other rheumatic diseases. Folate antagonism is known to contribute to the antiproliferative effects that are important in the action of methotrexate against malignant diseases, but concomitant administration of folic or folinic acid does not diminish the anti-inflammatory potential of this agent, which suggests that other mechanisms of action might be operative. Although no single mechanism is sufficient to account for all the anti-inflammatory activities of methotrexate, the release of adenosine from cells has been demonstrated both in vitro and in vivo. Methotrexate might also confer anti-inflammatory properties through the inhibition of polyamines. The biological effects on inflammation associated with adenosine release have provided insight into how methotrexate exerts its effects against inflammatory diseases and at the same time causes some of its well-known adverse effects. These activities contribute to the complex and multifaceted mechanisms that make methotrexate efficacious in the treatment of inflammatory disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Afane, M. et al. Discrepancy between 3H-thymidine uptake and cell cycle studies in stimulated lymphocyte cultures treated with methotrexate. Clin. Exp. Rheumatol. 7, 603–608 (1989).
van Ede, A. E. et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty-eight week, multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 44, 1515–1524 (2001).
Cronstein, B. N. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol. Rev. 57, 163–172 (2005).
Khanna, D. et al. Reduction of the efficacy of methotrexate by the use of folic acid: post hoc analysis from two randomized controlled studies. Arthritis Rheum. 52, 3030–3038 (2005).
Dervieux, T. et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Ann. Rheum. Dis. 64, 1180–1185 (2005).
Genestier, L. et al. Immunosuppressive properties of methotrexate: apoptosis and clonal deletion of activated peripheral T cells. J. Clin. Invest. 102, 322–328 (1998).
Olsen, N. J. & Murray, L. M. Antiproliferative effects of methotrexate on peripheral blood mononuclear cells. Arthritis Rheum. 32, 378–385 (1989).
Nesher, G., Moore, T. L. & Dorner, R. W. In vitro effects of methotrexate on peripheral blood monocytes: modulation by folinic acid and S-adenosylmethionine. Ann. Rheum. Dis. 50, 637–641 (1991).
Morabito, L. et al. Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J. Clin. Invest. 101, 295–300 (1998).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Peachell, P. T., Columbo, M., Kagey-Sobotka, A., Lichtenstein, L. M. & Marone, G. Adenosine potentiates mediator release from human lung mast cells. Am. Rev. Respir. Dis. 138, 1143–1151 (1988).
Green, P. G., Basbaum, A. I., Helms, C. & Levine, J. D. Purinergic regulation of bradykinin-induced plasma extravasation and adjuvant-induced arthritis in the rat. Proc. Natl Acad. Sci. USA 88, 4162–4165 (1991).
Cronstein, B. N., Naime, D. & Ostad, E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J. Clin. Invest. 92, 2675–2682 (1993).
Riksen, N. P. et al. Methotrexate modulates the kinetics of adenosine in humans in vivo. Ann. Rheum. Dis. 65, 465–470 (2006).
Dolezalova, P., Krijt, J., Chladek, J., Nemcova, D. & Hoza, J. Adenosine and methotrexate polyglutamate concentrations in patients with juvenile arthritis. Rheumatology (Oxford) 44, 74–79 (2005).
Cronstein, B. N., Kramer, S. B., Weissmann, G. & Hirschhorn, R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J. Exp. Med. 158, 1160–1177 (1983).
Cronstein, B. N. et al. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J. Immunology 148, 2201–2206 (1992).
Wakai, A. et al. Adenosine inhibits neutrophil vascular endothelial growth factor release and transendothelial migration via A2B receptor activation. Shock 15, 297–301 (2001).
Lennon, P. F., Taylor, C. T., Stahl, G. L. & Colgan, S. P. Neutrophil-derived 5′-adenosine monophosphate promotes endothelial barrier function via CD73-mediated conversion to adenosine and endothelial A2B receptor activation. J. Exp. Med. 188, 1433–1443 (1998).
Alarcon, G. S. et al. Suppression of rheumatoid factor production by methotrexate in patients with rheumatoid arthritis. Evidence for differential influences of therapy and clinical status on IgM and IgA rheumatoid factor expression. Arthritis Rheum. 33, 1156–1161 (1990).
Williams, A. S., Punn, Y. L., Amos, N., Cooper, A. M. & Williams, B. D. The effect of liposomally conjugated methotrexate upon mediator release from human peripheral blood monocytes. Br. J. Rheumatol. 34, 241–245 (1995).
Neurath, M. F. et al. Methotrexate specifically modulates cytokine production by T cells and macrophages in murine collagen-induced arthritis (CIA): a mechanism for methotrexate-mediated immunosuppression. Clin. Exp. Immunol. 115, 42–55 (1999).
Dolhain, R. J. et al. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Br. J. Rheumatol. 37, 502–508 (1998).
Seitz, M. et al. Methotrexate action in rheumatoid arthritis: stimulation of cytokine inhibitor and inhibition of chemokine production by peripheral blood mononuclear cells. Br. J. Rheumatol. 34, 602–609 (1995).
DiMartino, M. J., Johnson, W. J., Votta, B. & Hanna, N. Effect of antiarthritic drugs on the enhanced interleukin-1 (IL-1) production by macrophages from adjuvant-induced arthritic (AA) rats. Agents Actions 21, 348–350 (1987).
Segal, R., Mozes, E., Yaron, M. & Tartakovsky, B. The effects of methotrexate on the production and activity of interleukin-1. Arthritis Rheum. 32, 370–377 (1989).
Thomas, R. & Carroll, G. J. Reduction of leukocyte and interleukin-1 beta concentrations in the synovial fluid of rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 36, 1244–1252 (1993).
Sperling, R. I. et al. Inhibition of leukotriene B4 synthesis in neutrophils from patients with rheumatoid arthritis by a single oral dose of methotrexate. Arthritis Rheum. 33, 1149–1155 (1990).
Kremer, J. M., Galivan, J., Streckfuss, A. & Kamen, B. Methotrexate metabolism analysis in blood and liver of rheumatoid arthritis patients. Association with hepatic folate deficiency and formation of polyglutamates. Arthritis Rheum. 29, 832–835 (1986).
Puig, J. G. & Fox, I. H. Ethanol-induced activation of adenine nucleotide turnover. Evidence for a role of acetate. J. Clin. Invest. 74, 936–941 (1984).
Montesinos, M. C. et al. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am. J. Pathol. 160, 2009–2018 (2002).
Chan, E. S. et al. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br. J. Pharmacol. 148, 1144–1155 (2006).
Klatsky, A. L., Armstrong, M. A. & Friedman, G. D. Coffee, tea, and mortality. Ann. Epidemiol. 3, 375–381 (1993).
Nesher, G., Mates, M. & Zevin, S. Effect of caffeine consumption on efficacy of methotrexate in rheumatoid arthritis. Arthritis Rheum. 48, 571–572 (2003).
Merrill, J. T. et al. Adenosine A1 receptor promotion of multinucleated giant cell formation by human monocytes: a mechanism for methotrexate-induced nodulosis in rheumatoid arthritis. Arthritis Rheum. 40, 1308–1315 (1997).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Edwin S. L. Chan and Bruce N. Cronstein both declare that they hold patents pertinent to King Pharmaceuticals (on use of adenosine A2A receptor agonists to promote wound healing and use of A2A receptor antagonists to inhibit fibrosis; on the use of adenosine A1 receptor antagonists to treat osteoporosis and other diseases of bone; on the use of adenosine A1 and A2B receptor antagonists to treat fatty liver; and on the use of adenosine A2A receptor agonists to prevent prosthesis loosening). Bruce N. Cronstein declares that he has acted as a consultant for Cephalon, Cypress Bioscience, King Pharmaceuticals, CanFite Biopharma, Bristol-Myers Squibb, Cellzome, Takeda Pharmaceuticals, Prometheus Laboratories, Regeneron (Westat, DSMB), Sepracor, Amgen, Endocyte, Protalex, Allos, Combinatorx, Kyowa Hakka, Hoffman La Roche, Savient and Avidimer Therapeutics. Bruce N. Cronstein also declares he has stock in CanFite Biopharma, and has received grant or research support from King Pharmaceuticals, NIH and The Vilcek Foundation (of which he is a Board Member).
Rights and permissions
About this article
Cite this article
Chan, E., Cronstein, B. Methotrexate—how does it really work?. Nat Rev Rheumatol 6, 175–178 (2010). https://doi.org/10.1038/nrrheum.2010.5
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.5
This article is cited by
-
Calcification of Joints and Arteries (CALJA) Is a Rare Cause of Arthritis and Lower Limb Ischemia: Case Report and Literature Review
SN Comprehensive Clinical Medicine (2023)
-
Pharmacokinetics and bioequivalence evaluation of 2 oral formulations of methotrexate tablets in healthy Chinese volunteers under fasting and fed conditions
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
A multifunctional composite hydrogel as an intrinsic and extrinsic coregulator for enhanced therapeutic efficacy for psoriasis
Journal of Nanobiotechnology (2022)
-
Adenosine inhibits TNFα-induced MMP-3 production in MH7A rheumatoid arthritis synoviocytes via A2A receptor signaling
Scientific Reports (2022)
-
Effects of vitamin B12 on methotrexate hepatotoxicity: evaluation of receptor-interacting protein (RIP) kinase
Naunyn-Schmiedeberg's Archives of Pharmacology (2020)