Key Points
-
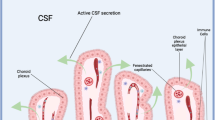
The choroid plexus (ChP) is a secretory tissue found in each of the brain ventricles, the main function of which is to produce cerebrospinal fluid (CSF). Although the ChP–CSF system is essential for proper development of the nervous system owing to fluid pressure within the ventricles as well as myriad CSF-borne signalling factors, it is nevertheless one of the most understudied areas of neurobiology.
-
A highly organized tissue, the ChP consists of simple cuboidal epithelial cells surrounding a core of fenestrated capillaries and connective tissue. As the interface between peripheral circulation and the CNS, the ChP forms the blood–CSF barrier via tight junctions between adjacent epithelial cells to restrict free passage of solutes from blood into CSF, and vice versa.
-
The ChP is present in chordates above the lancelet, and its development, which is classically categorized into four stages on the basis of its histological appearance, occurs in a stereotyped manner. Further, the order of ChP development seems to be conserved across species, with the hindbrain (fourth ventricle) ChP appearing first, followed by the bilateral appearance of the telencephalic (lateral ventricle) ChP, and the diencephalic (third ventricle) ChP appearing last.
-
The cell-intrinsic and -extrinsic molecular mechanisms that regulate ChP development are just now being elucidated. Although ChP epithelial cells are derived from neuroepithelial progenitors, they are non-neural cells in their mature state, suggesting the need to suppress neural character in favour of a non-neural cell fate.
-
Genetic fate-mapping studies have illustrated that cells contributing to the telencephalic ChP and hindbrain ChP exhibit lineage segregation in the mature tissues. Moreover, the ChPs are transcriptionally heterogeneous, a trait that appears to be evolutionarily conserved from mice to humans.
-
Recent work in the field has identified several ChP-derived factors with important roles in the developing and adult brain. Importantly, the ChP epithelial cell secretome has been described, suggesting a role for a ventricle-specific, regionalized CSF in the developing brain.
Abstract
The choroid plexus (ChP) is the principal source of cerebrospinal fluid (CSF), which has accepted roles as a fluid cushion and a sink for nervous system waste in vertebrates. Various animal models have provided insights into how the ChP–CSF system develops and matures. In addition, recent studies have uncovered new, active roles for this dynamic system in the regulation of neural stem cells, critical periods and the overall health of the nervous system. Together, these findings have brought about a paradigm shift in our understanding of brain development and health, and have stimulated new initiatives for the treatment of neurological disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others

References
Damkier, H. H., Brown, P. D. & Praetorius, J. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev. 93, 1847–1892 (2013).
Lehtinen, M. K. & Walsh, C. A. Neurogenesis at the brain–cerebrospinal fluid interface. Annu. Rev. Cell Dev. Biol. 27, 653–679 (2011).
Koh, L. et al. Development of cerebrospinal fluid absorption sites in the pig and rat: connections between the subarachnoid space and lymphatic vessels in the olfactory turbinates. Anat. Embryol. (Berl.) 211, 335–344 (2006).
Johnston, M., Zakharov, A., Koh, L. & Armstrong, D. Subarachnoid injection of Microfil reveals connections between cerebrospinal fluid and nasal lymphatics in the non-human primate. Neuropathol. Appl. Neurobiol. 31, 632–640 (2005).
Mollanji, R., Bozanovic-Sosic, R., Zakharov, A., Makarian, L. & Johnston, M. G. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1593–R1599 (2002).
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature http://dx.doi.org/10.1038/nature14432 (2015).
Lehtinen, M. K. et al. The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J. Neurosci. 33, 17553–17559 (2013).
Redzic, Z. B., Preston, J. E., Duncan, J. A., Chodobski, A. & Szmydynger-Chodobska, J. The choroid plexus–cerebrospinal fluid system: from development to aging. Curr. Top. Dev. Biol. 71, 1–52 (2005).
Johanson, C. E. et al. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 5, 10 (2008).
Brocklehurst, G. The significance of the evolution of the cerebrospinal fluid system. Ann. R. Coll. Surg. Engl. 61, 349–356 (1979).
Netsky, M. G. & Shuangshoti, S. The Choroid Plexus in Health and Disease (Univ. Press Virginia, 1975).
Zappaterra, M. D. et al. A comparative proteomic analysis of human and rat embryonic cerebrospinal fluid. J. Proteome Res. 6, 3537–3548 (2007).
Parada, C., Gato, A., Aparicio, M. & Bueno, D. Proteome analysis of chick embryonic cerebrospinal fluid. Proteomics 6, 312–320 (2006).
Desmond, M. E. & Jacobson, A. G. Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev. Biol. 57, 188–198 (1977).
Oi, S. Classification of hydrocephalus: critical analysis of classification categories and advantages of “Multi-categorical Hydrocephalus Classification” (Mc HC). Childs Nerv. Syst. 27, 1523–1533 (2011).
Shen, M. D. et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain 136, 2825–2835 (2013).
Wolburg, H. & Paulus, W. Choroid plexus: biology and pathology. Acta Neuropathol. 119, 75–88 (2010).
Zappaterra, M. W. & Lehtinen, M. K. The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell. Mol. Life Sci. 69, 2863–2878 (2012).
Emerich, D. F., Skinner, S. J., Borlongan, C. V., Vasconcellos, A. V. & Thanos, C. G. The choroid plexus in the rise, fall and repair of the brain. Bioessays 27, 262–274 (2005).
Zheng, W. & Chodobski, A. The Blood–Cerebrospinal Fluid Barrier (Taylor & Francis, 2005).
Dziegielewska, K. M., Ek, J., Habgood, M. D. & Saunders, N. R. Development of the choroid plexus. Microsc. Res. Tech. 52, 5–20 (2001).
Kappers, J. A. The development of the paraphysis cerebri in man with comments on its relationship to the intercolumnar tubercle and its significance for the origin of cystic tumors in the third ventricle. J. Comp. Neurol. 102, 425–509 (1955).
Currle, D. S., Cheng, X., Hsu, C. M. & Monuki, E. S. Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development 132, 3549–3559 (2005). This study reveals that most epithelial cells of the ChP differentiate from GDF7-expressing roof plate cells, with the exception of the posterior domains of the telencephalic ChP, which are induced by the roof plate in a non-cell-autonomous manner.
Liddelow, S. A., Dziegielewska, K. M., Vandeberg, J. L. & Saunders, N. R. Development of the lateral ventricular choroid plexus in a marsupial, Monodelphis domestica. Cerebrospinal Fluid Res. 7, 16 (2010).
Liddelow, S. A. et al. Molecular characterisation of transport mechanisms at the developing mouse blood–CSF interface: a transcriptome approach. PLoS ONE 7, e33554 (2012).
Kratzer, I. et al. Developmental changes in the transcriptome of the rat choroid plexus in relation to neuroprotection. Fluids Barriers CNS 10, 25 (2013).
Ek, C. J., Habgood, M. D., Dziegielewska, K. M. & Saunders, N. R. Structural characteristics and barrier properties of the choroid plexuses in developing brain of the opossum (Monodelphis domestica). J. Comp. Neurol. 460, 451–464 (2003).
Ek, C. J., Dziegielewska, K. M., Stolp, H. & Saunders, N. R. Functional effectiveness of the blood–brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica). J. Comp. Neurol. 496, 13–26 (2006).
Johansson, P. A. et al. Blood–CSF barrier function in the rat embryo. Eur. J. Neurosci. 24, 65–76 (2006).
Vogh, B. P. & Godman, D. R. Timolol plus acetazolamide: effect on formation of cerebrospinal fluid in cats and rats. Can. J. Physiol. Pharmacol. 63, 340–343 (1985).
Lindvall, M. & Owman, C. Autonomic nerves in the mammalian choroid plexus and their influence on the formation of cerebrospinal fluid. J. Cereb. Blood Flow Metab. 1, 245–266 (1981).
Ellis, D. Z., Nathanson, J. A. & Sweadner, K. J. Carbachol inhibits Na+–K+-ATPase activity in choroid plexus via stimulation of the NO/cGMP pathway. Am. J. Physiol. Cell Physiol. 279, C1685–C1693 (2000).
Redzic, Z. B. & Segal, M. B. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv. Drug Deliv. Rev. 56, 1695–1716 (2004).
Ransohoff, R. M. & Engelhardt, B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat. Rev. Immunol. 12, 623–635 (2012).
Lun, M. P. et al. Spatially heterogeneous choroid plexus transcriptomes encode positional identity and contribute to regional CSF production. J. Neurosci. 35, 4903–4916 (2015). This study demonstrates that telencephalic ChP and hindbrain ChP are heterogeneous tissues, distinct in their transcriptional profiles and positional identities, which translates functionally into the secretion of a regionalized CSF.
el-Gammal, S. The development of the diencephalic choroid plexus in the chick. A scanning electron-microscopic study. Cell Tissue Res. 219, 297–311 (1981).
el-Gammal, S. Regional surface changes during the development of the telencephalic choroid plexus in the chick. A scanning-electron microscopic study. Cell Tissue Res. 231, 251–263 (1983).
Thomas, T. & Dziadek, M. Capacity to form choroid plexus-like cells in vitro is restricted to specific regions of the mouse neural ectoderm. Development 117, 253–262 (1993).
Wilting, J. & Christ, B. An experimental and ultrastructural study on the development of the avian choroid plexus. Cell Tissue Res. 255, 487–494 (1989).
Hunter, N. L. & Dymecki, S. M. Molecularly and temporally separable lineages form the hindbrain roof plate and contribute differentially to the choroid plexus. Development 134, 3449–3460 (2007). This study reveals that the rhombic lip as well as distinct spatiotemporal fields of the hindbrain roof plate differentially contribute to hindbrain ChP development.
Huang, X. et al. Sonic hedgehog signaling regulates a novel epithelial progenitor domain of the hindbrain choroid plexus. Development 136, 2535–2543 (2009). This study identifies a SHH-responsive progenitor domain adjacent to the lower rhombic lip that regulates progenitor proliferation and hindbrain ChP development.
Li, Y., Chen, J. & Chopp, M. Cell proliferation and differentiation from ependymal, subependymal and choroid plexus cells in response to stroke in rats. J. Neurol. Sci. 193, 137–146 (2002).
Barkho, B. Z. & Monuki, E. S. Proliferation of cultured mouse choroid plexus epithelial cells. PLoS ONE 10, e0121738 (2015).
Safaee, M. et al. Choroid plexus papillomas: advances in molecular biology and understanding of tumorigenesis. Neuro Oncol. 15, 255–267 (2013).
Imayoshi, I., Shimogori, T., Ohtsuka, T. & Kageyama, R. Hes genes and neurogenin regulate non-neural versus neural fate specification in the dorsal telencephalic midline. Development 135, 2531–2541 (2008).
Hebert, J. M., Mishina, Y. & McConnell, S. K. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron 35, 1029–1041 (2002). This study demonstrates that BMP signalling at the dorsal midline has a crucial role in instructing the specification and differentiation of the telencephalic ChP.
Chizhikov, V. V. et al. Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proc. Natl Acad. Sci. USA 107, 10725–10730 (2010). This study identifies LMX1A as critical for the development of the roof plate and subsequent specification of dorsal cell fates in the developing CNS.
Millonig, J. H., Millen, K. J. & Hatten, M. E. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature 403, 764–769 (2000).
Monuki, E. S., Porter, F. D. & Walsh, C. A. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate–Lhx2 pathway. Neuron 32, 591–604 (2001).
von Frowein, J., Wizenmann, A. & Gotz, M. The transcription factors Emx1 and Emx2 suppress choroid plexus development and promote neuroepithelial cell fate. Dev. Biol. 296, 239–252 (2006).
Johansson, P. A. et al. The transcription factor Otx2 regulates choroid plexus development and function. Development 140, 1055–1066 (2013).
Nicholson-Flynn, K., Hitchcock-DeGregori, S. E. & Levitt, P. Restricted expression of the actin-regulatory protein, tropomyosin, defines distinct boundaries, evaginating neuroepithelium, and choroid plexus forerunners during early CNS development. J. Neurosci. 16, 6853–6863 (1996).
Awatramani, R., Soriano, P., Rodriguez, C., Mai, J. J. & Dymecki, S. M. Cryptic boundaries in roof plate and choroid plexus identified by intersectional gene activation. Nat. Genet. 35, 70–75 (2003). This study developed a combinatorial recombinase-based method for fate mapping cells, revealing that the hindbrain roof plate originates from Wnt1 -expressing rhombencephalic neuroectoderm, which gives rise to the hindbrain ChP, and that these structures develop in a patterned, segmental manner.
Landsberg, R. L. et al. Hindbrain rhombic lip is comprised of discrete progenitor cell populations allocated by Pax6. Neuron 48, 933–947 (2005).
Sturrock, R. R. A morphological study of the development of the mouse choroid plexus. J. Anat. 129, 777–793 (1979).
Chizhikov, V. V. et al. The roof plate regulates cerebellar cell-type specification and proliferation. Development 133, 2793–2804 (2006).
Lobas, M. A. et al. Molecular heterogeneity in the choroid plexus epithelium: the 22-member γ-protocadherin family is differentially expressed, apically localized, and implicated in CSF regulation. J. Neurochem. 120, 913–927 (2012).
Quay, W. B. Regional differences in metabolism and composition of choroid plexuses. Brain Res. 2, 378–389 (1966).
Nathanson, J. A. β-adrenergic-sensitive adenylate cyclase in choroid plexus: properties and cellular localization. Mol. Pharmacol. 18, 199–209 (1980).
Irvin, D. K., Nakano, I., Paucar, A. & Kornblum, H. I. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J. Neurosci. Res. 75, 330–343 (2004).
Garcia-Lecea, M., Kondrychyn, I., Fong, S. H., Ye, Z. R. & Korzh, V. In vivo analysis of choroid plexus morphogenesis in zebrafish. PLoS ONE 3, e3090 (2008).
Bill, B. R. et al. Development and Notch signaling requirements of the zebrafish choroid plexus. PLoS ONE 3, e3114 (2008).
Dang, L. et al. Notch3 signaling initiates choroid plexus tumor formation. Oncogene 25, 487–491 (2006).
Pierfelice, T. J. et al. Notch3 activation promotes invasive glioma formation in a tissue site-specific manner. Cancer Res. 71, 1115–1125 (2011).
Knudsen, P. A. Mode of growth of the choroid plexus in mouse embryos. Acta Anat. (Basel) 57, 172–182 (1964).
Tennyson, V. M. & Pappas, G. D. Fine structure of the developing telencephalic and myelencephalic choroid plexus in the rabbit. J. Comp. Neurol. 123, 379–411 (1964).
Furuta, Y., Piston, D. W. & Hogan, B. L. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 124, 2203–2212 (1997).
Watanabe, M. et al. BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors. J. Neurosci. 32, 15934–15945 (2012). This study found that BMP4 is sufficient to induce ChP epithelial cells from embryonic stem cell-derived neuroepithelial cells, which hold tremendous promise for the treatment of neurological diseases.
Cheng, X. et al. Central roles of the roof plate in telencephalic development and holoprosencephaly. J. Neurosci. 26, 7640–7649 (2006).
Panchision, D. M. et al. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 15, 2094–2110 (2001).
Fernandes, M., Gutin, G., Alcorn, H., McConnell, S. K. & Hebert, J. M. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development 134, 3789–3794 (2007).
Bailey, P. Morphology of the roof plate of the forebrain and the lateral choroid plexuses in the human embryo. J. Comp. Neurol. 26, 79–120 (1915).
Grove, E. A., Tole, S., Limon, J., Yip, L. & Ragsdale, C. W. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 125, 2315–2325 (1998).
Louvi, A., Yoshida, M. & Grove, E. A. The derivatives of the Wnt3a lineage in the central nervous system. J. Comp. Neurol. 504, 550–569 (2007).
Shimogori, T., Banuchi, V., Ng, H. Y., Strauss, J. B. & Grove, E. A. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development 131, 5639–5647 (2004).
Franz, T. Extra-toes (Xt) homozygous mutant mice demonstrate a role for the Gli-3 gene in the development of the forebrain. Acta Anat. (Basel) 150, 38–44 (1994).
Theil, T., Alvarez-Bolado, G., Walter, A. & Ruther, U. Gli3 is required for Emx gene expression during dorsal telencephalon development. Development 126, 3561–3571 (1999).
Konno, D. et al. The mammalian DM domain transcription factor Dmrta2 is required for early embryonic development of the cerebral cortex. PLoS ONE 7, e46577 (2012).
Porter, F. D. et al. Lhx2, a LIM homeobox gene, is required for eye, forebrain, and definitive erythrocyte development. Development 124, 2935–2944 (1997).
Shuangshoti, S. & Netsky, M. G. Histogenesis of choroid plexus in man. Am. J. Anat. 118, 283–316 (1966).
Dohrmann, G. J. The choroid plexus: a historical review. Brain Res. 18, 197–218 (1970).
Keep, R. F. & Jones, H. C. A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res. Dev. Brain Res. 56, 47–53 (1990).
Quinton, P. M., Wright, E. M. & Tormey, J. M. Localization of sodium pumps in the choroid plexus epithelium. J. Cell Biol. 58, 724–730 (1973).
Serot, J. M., Foliguet, B., Bene, M. C. & Faure, G. C. Choroid plexus and ageing in rats: a morphometric and ultrastructural study. Eur. J. Neurosci. 14, 794–798 (2001).
Sturrock, R. R. An ultrastructural study of the choroid plexus of aged mice. Anat. Anz. 165, 379–385 (1988).
Banizs, B. et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132, 5329–5339 (2005).
Banizs, B. et al. Altered pHi regulation and Na+/HCO3− transporter activity in choroid plexus of cilia-defective Tg737orpk mutant mouse. Am. J. Physiol. Cell Physiol. 292, C1409–C1416 (2007).
Swiderski, R. E. et al. Structural defects in cilia of the choroid plexus, subfornical organ and ventricular ependyma are associated with ventriculomegaly. Fluids Barriers CNS 9, 22 (2012).
Narita, K., Kawate, T., Kakinuma, N. & Takeda, S. Multiple primary cilia modulate the fluid transcytosis in choroid plexus epithelium. Traffic 11, 287–301 (2010).
Broom, E. R., Gilthorpe, J. D., Butts, T., Campo-Paysaa, F. & Wingate, R. J. The roof plate boundary is a bi-directional organiser of dorsal neural tube and choroid plexus development. Development 139, 4261–4270 (2012).
Nielsen, C. M. & Dymecki, S. M. Sonic hedgehog is required for vascular outgrowth in the hindbrain choroid plexus. Dev. Biol. 340, 430–437 (2010).
Cushing, H. Studies on the cerebro-spinal fluid: I. Introduction. J. Med. Res. 31, 1–19 (1914).
Dandy, W. E. Experimental hydrocephalus. Ann. Surg. 70, 129–142 (1919).
Dandy, W. E. & Blackfan, K. D. An experimental and clinical study of internal hydrocephalus. J. Am. Med. Associ. 61, 2216–2217 (1913).
Johansson, P. A. et al. Aquaporin-1 in the choroid plexuses of developing mammalian brain. Cell Tissue Res. 322, 353–364 (2005).
Liddelow, S. A. et al. Cellular transfer of macromolecules across the developing choroid plexus of Monodelphis domestica. Eur. J. Neurosci. 29, 253–266 (2009).
Ek, C. J. et al. Efflux mechanisms at the developing brain barriers: ABC-transporters in the fetal and postnatal rat. Toxicol. Lett. 197, 51–59 (2010).
Brown, P. D., Davies, S. L., Speake, T. & Millar, I. D. Molecular mechanisms of cerebrospinal fluid production. Neuroscience 129, 957–970 (2004).
Bass, N. H. & Lundborg, P. Postnatal development of bulk flow in the cerebrospinal fluid system of the albino rat: clearance of carboxyl-[14C]inulin after intrathecal infusion. Brain Res. 52, 323–332 (1973).
Liddelow, S. A. et al. Modification of protein transfer across blood/cerebrospinal fluid barrier in response to altered plasma protein composition during development. Eur. J. Neurosci. 33, 391–400 (2011).
Abbott, G. W. et al. KCNQ1, KCNE2, and Na+-coupled solute transporters form reciprocally regulating complexes that affect neuronal excitability. Sci. Signal. 7, ra22 (2014).
Dziegielewska, K. M. et al. Proteins in cerebrospinal fluid and plasma of fetal sheep during development. J. Physiol. 300, 441–455 (1980).
Lehtinen, M. K. et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69, 893–905 (2011). This study showed that secreted factors in the CSF, including ChP-secreted IGF2, regulate cerebral cortical progenitor cell proliferation and brain development.
Ek, C. J., Dziegielewska, K. M. & Saunders, N. R. in Development of the Blood–Cerebrospinal Fluid Barrier (eds Zheng, W. & Chodobski, A.) 3–23 (Taylor & Francis, 2005).
Grapp, M. et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat. Commun. 4, 2123 (2013).
Feliciano, D. M., Zhang, S., Nasrallah, C. M., Lisgo, S. N. & Bordey, A. Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification. PLoS ONE 9, e88810 (2014).
Tietje, A., Maron, K. N., Wei, Y. & Feliciano, D. M. Cerebrospinal fluid extracellular vesicles undergo age dependent declines and contain known and novel non-coding RNAs. PLoS ONE 9, e113116 (2014).
Nonami, Y., Narita, K., Nakamura, H., Inoue, T. & Takeda, S. Developmental changes in ciliary motility on choroid plexus epithelial cells during the perinatal period. Cytoskeleton (Hoboken) 70, 797–803 (2013).
Louvi, A. & Grove, E. A. Cilia in the CNS: the quiet organelle claims center stage. Neuron 69, 1046–1060 (2011).
Zappaterra, M. W., LaMantia, A. S., Walsh, C. A. & Lehtinen, M. K. Isolation of cerebrospinal fluid from rodent embryos for use with dissected cerebral cortical explants. J. Vis. Exp. 73, e50333 (2013).
Higginbotham, H. et al. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nat. Neurosci. 16, 1000–1007 (2013).
Yeh, C. et al. IGF-1 activates a cilium-localized noncanonical Gβγ signaling pathway that regulates cell-cycle progression. Dev. Cell 26, 358–368 (2013).
Martin, C. et al. FGF2 plays a key role in embryonic cerebrospinal fluid trophic properties over chick embryo neuroepithelial stem cells. Dev. Biol. 297, 402–416 (2006).
Huang, X. et al. Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc. Natl Acad. Sci. USA 107, 8422–8427 (2010).
Yamamoto, M., McCaffery, P. & Drager, U. C. Influence of the choroid plexus on cerebellar development: analysis of retinoic acid synthesis. Brain Res. Dev. Brain Res. 93, 182–190 (1996).
Chang, J. T., Lehtinen, M. K. & Sive, H. Zebrafish cerebrospinal fluid mediates cell survival through a retinoid signaling pathway. Dev. Neurobiol. http://dx.doi.org/10.1002/dneu.22300 (2015).
Hatta, T. et al. Quantitative analyses of leukemia inhibitory factor in the cerebrospinal fluid in mouse embryos. Neuroreport 17, 1863–1866 (2006).
Gregg, C. & Weiss, S. CNTF/LIF/gp130 receptor complex signaling maintains a VZ precursor differentiation gradient in the developing ventral forebrain. Development 132, 565–578 (2005).
Arbeille, E. et al. Cerebrospinal fluid-derived Semaphorin3B orients neuroepithelial cell divisions in the apicobasal axis. Nat. Commun. 6, 6366 (2015).
Cavanagh, M. E. et al. Comparison of proteins in CSF of lateral and IVth ventricles during early development of fetal sheep. Brain Res. 313, 159–167 (1983).
Puelles, L. & Rubenstein, J. L. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 16, 472–479 (1993).
Philippidou, P. & Dasen, J. S. Hox genes: choreographers in neural development, architects of circuit organization. Neuron 80, 12–34 (2013).
Mirzadeh, Z., Merkle, F. T., Soriano-Navarro, M., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3, 265–278 (2008).
Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Fuentealba, L. C., Obernier, K. & Alvarez-Buylla, A. Adult neural stem cells bridge their niche. Cell Stem Cell 10, 698–708 (2012).
Kokovay, E. et al. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell 11, 220–230 (2012).
Delgado, A. C. et al. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 83, 572–585 (2014). This study showed that NT3 secreted by both brain and ChP capillaries regulates adult neurogenesis.
Sawamoto, K. et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 311, 629–632 (2006). This study identified gradients of CSF proteins — in particular, SLIT — that influence the migration of newly born adult neurons.
Baruch, K. et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93 (2014). This study identified an ageing-induced IFN response in the ChP and that the modulation of this response can influence cognitive function.
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Katsimpardi, L. et al. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634 (2014).
Sugiyama, S. et al. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134, 508–520 (2008).
Spatazza, J. et al. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 3, 1815–1823 (2013). This study identified the ChP as the source of OTX2, which is taken up by interneurons in the forebrain to regulate binocular vision and critical periods.
Toda, T. et al. Birth regulates the initiation of sensory map formation through serotonin signaling. Dev. Cell 27, 32–46 (2013).
Nickel, W. & Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10, 148–155 (2009).
Xie, L. et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl Med. 4, 147ra111 (2012). Using in vivo two-photon imaging of superficial regions in the cerebral cortex, this study showed CSF entry into the brain along para-arterial spaces, whereas interstitial fluid and soluble amyloid-β were cleared along the brain's paravenous pathways, all in an astrocyte- and aquaporin 4-dependent manner.
Iliff, J. J. et al. Cerebral arterial pulsation drives paravascular CSF–interstitial fluid exchange in the murine brain. J. Neurosci. 33, 18190–18199 (2013).
Henson, H. E., Parupalli, C., Ju, B. & Taylor, M. R. Functional and genetic analysis of choroid plexus development in zebrafish. Front. Neurosci. 8, 364 (2014).
Prinz, M. & Priller, J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 15, 300–312 (2014).
Monier, A., Evrard, P., Gressens, P. & Verney, C. Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J. Comp. Neurol. 499, 565–582 (2006).
Cunningham, C. L., Martinez-Cerdeno, V. & Noctor, S. C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33, 4216–4233 (2013).
Tremblay, M. E. et al. The role of microglia in the healthy brain. J. Neurosci. 31, 16064–16069 (2011).
Pont-Lezica, L., Bechade, C., Belarif-Cantaut, Y., Pascual, O. & Bessis, A. Physiological roles of microglia during development. J. Neurochem. 119, 901–908 (2011).
Steffen, B. J., Breier, G., Butcher, E. C., Schulz, M. & Engelhardt, B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am. J. Pathol. 148, 1819–1838 (1996).
Kunis, G. et al. IFN-γ-dependent activation of the brain's choroid plexus for CNS immune surveillance and repair. Brain 136, 3427–3440 (2013).
Schwartz, M. & Baruch, K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 33, 7–22 (2014).
Shechter, R. et al. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38, 555–569 (2013).
Shechter, R., London, A. & Schwartz, M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat. Rev. Immunol. 13, 206–218 (2013).
Demasio, K. et al. Isolated choroid plexus cyst in low-risk women less than 35 years old. Am. J. Obstet. Gynecol. 187, 1246–1249 (2002).
Greenfield, J. G., Love, S., Louis, D. N. & Ellison, D. Greenfield's Neuropathology (Hodder Arnold, 2008).
Tavani, F., Zimmerman, R. A., Clancy, R. R., Licht, D. J. & Mahle, W. T. Incidental intracranial hemorrhage after uncomplicated birth: MRI before and after neonatal heart surgery. Neuroradiology 45, 253–258 (2003).
van Rybroek, J. J. & Moore, S. A. Sudden death from choroid plexus vascular malformation hemorrhage: case report and review of the literature. Clin. Neuropathol. 9, 39–45 (1990).
Naeini, R. M., Yoo, J. H. & Hunter, J. V. Spectrum of choroid plexus lesions in children. AJR Am. J. Roentgenol. 192, 32–40 (2009).
Tong, Y. et al. Cross-species genomics identifies TAF12, NFYC, and RAD54L as choroid plexus carcinoma oncogenes. Cancer Cell 27, 712–727 (2015).
Hirano, H. et al. Hydrocephalus due to villous hypertrophy of the choroid plexus in the lateral ventricles. Case report. J. Neurosurg. 80, 321–323 (1994).
Fujimura, M. et al. Hydrocephalus due to cerebrospinal fluid overproduction by bilateral choroid plexus papillomas. Childs Nerv. Syst. 20, 485–488 (2004).
Smith, Z. A. et al. Choroid plexus hyperplasia: surgical treatment and immunohistochemical results. Case report. J. Neurosurg. 107, 255–262 (2007).
Davis, J. D. & Tremont, G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 32, 49–65 (2007).
Davis, J. D., Stern, R. A. & Flashman, L. A. Cognitive and neuropsychiatric aspects of subclinical hypothyroidism: significance in the elderly. Curr. Psychiatry Rep. 5, 384–390 (2003).
Fleming, C. E., Nunes, A. F. & Sousa, M. M. Transthyretin: more than meets the eye. Prog. Neurobiol. 89, 266–276 (2009).
Serot, J. M., Bene, M. C. & Faure, G. C. Choroid plexus, aging of the brain, and Alzheimer's disease. Front. Biosci. 8, s515–s521 (2003).
Maurizi, C. P. Choroid plexus portals and a deficiency of melatonin can explain the neuropathology of Alzheimer's disease. Med. Hypotheses 74, 1059–1066 (2010).
Silverberg, G. D., Mayo, M., Saul, T., Rubenstein, E. & McGuire, D. Alzheimer's disease, normal-pressure hydrocephalus, and senescent changes in CSF circulatory physiology: a hypothesis. Lancet Neurol. 2, 506–511 (2003).
Haddad, M. R., Donsante, A., Zerfas, P. & Kaler, S. G. Fetal brain-directed AAV gene therapy results in rapid, robust, and persistent transduction of mouse choroid plexus epithelia. Mol. Ther. Nucleic Acids 2, e101 (2013).
Haskell, R. E., Hughes, S. M., Chiorini, J. A., Alisky, J. M. & Davidson, B. L. Viral-mediated delivery of the late-infantile neuronal ceroid lipofuscinosis gene, TPP-I to the mouse central nervous system. Gene Ther. 10, 34–42 (2003).
Hladky, S. B. & Barrand, M. A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11, 26 (2014).
Acknowledgements
The authors thank members of the Lehtinen and Monuki laboratories for helpful discussions. The authors apologize to investigators whose work they could not reference owing to space limitations. This work was supported by CIRM RN2-00915-1 and UCI ICTS and ADRC Pilot Project Awards (to E.S.M.); and Pediatric Hydrocephalus Foundation, Alfred P. Sloan Foundation, NIH K99/R00 NS072192 and R01 NS088566 (to M.K.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Cribriform plate
-
The region of skull bone supporting the olfactory bulbs, which is perforated to allow the passage of olfactory nerves from the nasal cavities.
- Hydrocephalus
-
A condition resulting from excess accumulation of cerebrospinal fluid within the ventricles of the brain.
- Neuroepithelial cells
-
The stem cells of the nervous system, these cells initially undergo symmetric division early in development to expand the progenitor cell pool, and subsequently give rise to more lineage-restricted cells at the start of neurogenesis, including radial glial and choroid plexus epithelial cells.
- Rhombic lip
-
A transient structure located at the interface between the roof plate and dorsal neuroepithelium, which functions as a germinal epithelium and a source of diffusible signals.
- Cortical hem
-
A signalling centre located bilaterally near the telencephalic midline, which functions as a source of WNTs and bone morphogenetic proteins for cerebral cortical, hippocampal and choroid plexus development, as well as a source of Cajal–Retzius cells.
- Prosomeres
-
Segments of the anterior neural tube early in embryonic development that give rise to forebrain structures.
- Rhombomeres
-
Transient, regularly spaced repeating units of hindbrain cells in the developing embryo that will ultimately give rise to the rhombencephalon.
- Trisomy 18
-
A genetic condition in which cells contain three copies of chromosome 18 rather than the normal two. It is also known as Edwards syndrome.
- Aicardi syndrome
-
A developmental disorder that is characterized by infantile spasms and defects of the corpus callosum and eyes (chorioretinopathy); see Online Mendelian Inheritance in Man 304050.
- Bromodeoxyuridine
-
(BrdU). A synthetic thymidine analogue that can be incorporated into replicating DNA.
- Choroid plaque
-
The non-papillary tissue at the immediate dorsal midline that separates the two choroid plexi of the lateral ventricles.
- Fenestrated capillaries
-
Openings in the endothelium are bridged by thin diaphragms permeable to water and small molecules.
Rights and permissions
About this article
Cite this article
Lun, M., Monuki, E. & Lehtinen, M. Development and functions of the choroid plexus–cerebrospinal fluid system. Nat Rev Neurosci 16, 445–457 (2015). https://doi.org/10.1038/nrn3921
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3921