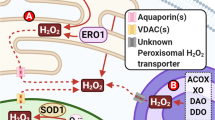
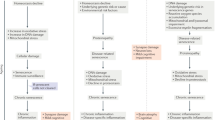
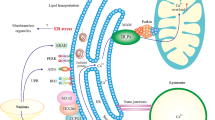
Abstract
Oxidative stress has long been linked to the neuronal cell death that is associated with certain neurodegenerative conditions. Whether it is a primary cause or merely a downstream consequence of the neurodegenerative process is still an open question, however. The advent of a growing number of in vitro and in vivo models that emulate human disease pathology is aiding scientists in deciphering just where oxidative stress intersects with other cellular events in the emerging roadmap leading to neurodegeneration. Here I review the evidence for oxidative stress in neurodegeneration and how this relates to other cellular events.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hensley, K. et al. Electrochemical analysis of protein nitrotyrosine and dityrosine in the Alzheimer brain indicates region-specific accumulation. J. Neurosci. 18, 8126–8132 (1998).
Butterfield, D.A., Castegna, A., Lauderback, C.M. & Drake, J. Evidence that amyloid β-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol. Aging 23, 655–664 (2002).
Dexter, D.T. et al. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J. Neurochem. 52, 381–389 (1989).
Pedersen, W.A. et al. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann. Neurol. 44, 819–824 (1998).
Beal, M.F. et al. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 42, 644–654 (1997).
Smith, M.A., Richey Harris, P.L., Sayre, L.M., Beckman, J.S. & Perry, G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J. Neurosci. 17, 2653–2657 (1997).
Good, P.F., Hsu, A., Werner, P., Perl, D.P. & Olanow, C.W. Protein nitration in Parkinson's disease. J. Neuropathol. Exp. Neurol. 57, 338–342 (1998).
Aoyama, K. et al. Nitration of manganese superoxide dismutase in cerebrospinal fluids is a marker for peroxynitrite-mediated oxidative stress in neurodegenerative diseases. Ann. Neurol. 47, 524–527 (2000).
Giasson, B.I. et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science 290, 985–989 (2000).
Giasson, B.I. et al. A panel of epitope-specific antibodies detects protein domains distributed throughout human α-synuclein in Lewy bodies of Parkinson's disease. J. Neurosci. Res. 59, 528–533 (2000).
Horiguchi, T. et al. Nitration of tau protein is linked to neurodegeneration in tauopathies. Am. J. Pathol. 163, 1021–1031 (2003).
Zemlan, F.P., Thienhaus, O.J. & Bosmann, H.B. Superoxide dismutase activity in Alzheimer's disease: possible mechanism for paired helical filament formation. Brain Res. 476, 160–162 (1989).
Pappolla, M.A., Omar, R.A., Kim, K.S. & Robakis, N.K. Immunohistochemical evidence of oxidative stress in Alzheimer's disease. Am. J. Pathol. 140, 621–628 (1992).
Gabbita, S.P., Aksenov, M.Y., Lovell, M.A. & Markesbery, W.R. Decrease in peptide methionine sulfoxide reductase in Alzheimer's disease brain. J. Neurochem. 73, 1660–1666 (1999).
Perry, T.L., Godin, D.V. & Hansen, S. Parkinson's disease: a disorder due to nigral glutathione deficiency? Neurosci. Lett. 33, 305–310 (1982).
Perry, TL. & Yong, V.W. Idiopathic Parkinson's disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci. Lett. 67, 269–274 (1986).
Pearce, R.K., Owen, A., Daniel, S., Jenner, P. & Marsden, C.D. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. J. Neural Transm. 104, 661–677 (1997).
Riederer, P. et al. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 52, 515–520 (1989).
Sofic, E., Paulus, W., Jellinger, K., Riederer, P. & Youdim, M.B. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J. Neurochem. 56, 978–982 (1991).
Jellinger, K.A. et al. Iron and ferritin in substantia nigra in Parkinson's disease. Adv. Neurol. 60, 267–272 (1993).
Cudkowicz, M.E. et al. Epidemiology of mutations in superoxide dismutase in amyotrophic lateral sclerosis. Ann. Neurol. 41, 210–221 (1997).
Gurney, M.E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Hall, E.D., Andrus, P.K., Oostveen, J.A., Fleck, T.J. & Gurney, M.E. Relationship of oxygen radical-induced lipid peroxidative damage to disease onset and progression in a transgenic model of familial ALS. J. Neurosci. Res. 53, 66–77 (1998).
Warita, H., Hayashi, T., Murakami, T., Manabe, Y. & Abe, K. Oxidative damage to mitochondrial DNA in spinal motoneurons of transgenic ALS mice. Brain Res. Mol. Brain Res. 89, 147–152 (2001).
Dal Canto, M.C. Comparison of pathological alterations in ALS and a murine transgenic model: pathogenetic implications. Clin. Neurosci. 3, 332–337 (1995).
Zhang, J., Graham, D.G., Montine, T.J. & Ho, Y.S. Enhanced N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in mice deficient in CuZn-superoxide dismutase or glutathione peroxidase. J. Neuropathol. Exp. Neurol. 59, 53–61 (2000).
Klivenyi, P. et al. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J. Neurosci. 20, 1–7 (2000).
Przedborski, S. et al. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J. Neurosci. 12, 1658–1667 (1992).
Andreassen, O.A. et al. Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Exp. Neurol. 167, 189–195 (2001).
Klivenyi, P. et al. Manganese superoxide dismutase overexpression attenuates MPTP toxicity. Neurobiol. Dis. 5, 253–258 (1998).
Klivenyi, P. et al. Inhibition of neuronal nitric oxide synthase protects against MPTP toxicity. Neuroreport 11, 1265–1268 (2000).
Liberatore, G.T. et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 5, 1403–1409 (1999).
Dehmer, T., Lindenau, J., Haid, S., Dichgans, J. & Schulz, J.B. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J. Neurochem. 74, 2213–2216 (2000).
Itzhak, Y., Martin, J.L. & Ali, S.F. Methamphetamine- and 1-methyl-4-phenyl- 1,2,3, 6-tetrahydropyridine-induced dopaminergic neurotoxicity in inducible nitric oxide synthase–deficient mice. Synapse 34, 305–312 (1999).
Zhu, S. et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417, 74–78 (2002).
Wu, D.C. et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 22, 1763–1771 (2002).
Du, Y. et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 98, 14669–14674 (2001).
Yang, L. et al. Minocycline enhances MPTP toxicity to dopaminergic neurons. J. Neurosci. Res. 74, 278–285 (2003).
Sano, M. et al. A controlled trial of selegiline, α-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N. Engl. J. Med. 336, 1216–1222 (1997).
Effects of tocopherol and deprenyl on the progression of disability in early Parkinson's disease. The Parkinson Study Group. N. Engl. J. Med. 328, 176–183 (1993).
Desnuelle, C., Dib, M., Garrel, C. & Favier, A. A double-blind, placebo-controlled randomized clinical trial of α-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. ALS riluzole-tocopherol Study Group. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2, 9–18 (2001).
Gurney, M.E., Fleck, T.J., Himes, C.S. & Hall, E.D. Riluzole preserves motor function in a transgenic model of familial amyotrophic lateral sclerosis. Neurology 50, 62–66 (1998).
Gurney, M.E. et al. Benefit of vitamin E, riluzole, and gabapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann. Neurol. 39, 147–157 (1996).
Kieran, D. et al. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat. Med. 10, 402–405 (2004).
Winklhofer, K.F., Henn, I.H., Kay-Jackson, P.C., Heller, U. & Tatzelt, J. Inactivation of parkin by oxidative stress and C-terminal truncations: a protective role of molecular chaperones. J. Biol. Chem. 278, 47199–47208 (2003).
Bruening, W. et al. Up-regulation of protein chaperones preserves viability of cells expressing toxic Cu/Zn-superoxide dismutase mutants associated with amyotrophic lateral sclerosis. J. Neurochem. 72, 693–699 (1999).
Takeuchi, H. et al. Hsp70 and Hsp40 improve neurite outgrowth and suppress intracytoplasmic aggregate formation in cultured neuronal cells expressing mutant SOD1. Brain Res. 949, 11–22 (2002).
Warrick, J.M. et al. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat. Genet. 23, 425–428 (1999).
Manning-Bog, A.B., McCormack, A.L., Purisai, M.G., Bolin, L.M. & Di Monte, D.A. α-Synuclein overexpression protects against paraquat-induced neurodegeneration. J. Neurosci. 23, 3095–3099 (2003).
Conway, K.A., Rochet, J.C., Bieganski, R.M. & Lansbury, P.T., Jr. Kinetic stabilization of the α-synuclein protofibril by a dopamine–α-synuclein adduct. Science 294, 1346–1349 (2001).
Volles, M.J. & Lansbury, P.T., Jr. Vesicle permeabilization by protofibrillar α-synuclein is sensitive to Parkinson's disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 41, 4595–4602 (2002).
Lotharius, J. & Brundin, P. Impaired dopamine storage resulting from α-synuclein mutations may contribute to the pathogenesis of Parkinson's disease. Hum. Mol. Genet. 11, 2395–2407 (2002).
Xu, J. et al. Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 8, 600–606 (2002).
Abeliovich, A. et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252 (2000).
Jha, N., Kumar, M.J., Boonplueang, R. & Andersen, J.K. Glutathione decreases in dopaminergic PC12 cells interfere with the ubiquitin protein degradation pathway: relevance for Parkinson's disease? J. Neurochem. 80, 555–561 (2002).
Friguet, B. & Szweda, L.I. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 405, 21–25 (1997).
Okada, K. et al. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J. Biol. Chem. 274, 23787–23793 (1999).
Rakhit, R. et al. Monomeric Cu,Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J. Biol. Chem. 279, 15499–15504 (2004).
Behl, C., Davis, J.B., Lesley, R. & Schubert, D. Hydrogen peroxide mediates amyloid-β protein toxicity. Cell 77, 817–827 (1994).
Hsu, L.J. et al. α-Synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 157, 401–410 (2000).
Ostrerova-Golts, N. et al. The A53T α-synuclein mutation increases iron-dependent aggregation and toxicity. J. Neurosci. 20, 6048–6054 (2000).
Hyun, D.H. et al. Effect of wild-type or mutant Parkin on oxidative damage, nitric oxide, antioxidant defenses, and the proteasome. J. Biol. Chem. 277, 28572–28577 (2002).
Lee, M., Hyun, D., Jenner, P. & Halliwell, B. Effect of overexpression of wild-type and mutant Cu/Zn-superoxide dismutases on oxidative damage and antioxidant defences: relevance to Down's syndrome and familial amyotrophic lateral sclerosis. J. Neurochem. 76, 957–965 (2001).
Lee, M., Hyun, D.H., Halliwell, B. & Jenner, P. Effect of overexpression of wild-type and mutant Cu/Zn-superoxide dismutases on oxidative stress and cell death induced by hydrogen peroxide, 4-hydroxynonenal or serum deprivation: potentiation of injury by ALS-related mutant superoxide dismutases and protection by Bcl-2. J. Neurochem. 78, 209–220 (2001).
Kruman, I.I., Pedersen, W.A., Springer, J.E. & Mattson, M.P. ALS-linked Cu/Zn-SOD mutation increases vulnerability of motor neurons to excitotoxicity by a mechanism involving increased oxidative stress and perturbed calcium homeostasis. Exp. Neurol. 160, 28–39 (1999).
Xie, Z. et al. Peroxynitrite mediates neurotoxicity of amyloid β-peptide1–42- and lipopolysaccharide-activated microglia. J. Neurosci. 22, 3484–3492 (2002).
McNaught, K.S. & Jenner, P. Altered glial function causes neuronal death and increases neuronal susceptibility to 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced toxicity in astrocytic/ventral mesencephalic co-cultures. J. Neurochem. 73, 2469–2476 (1999).
Knott, C., Stern, G. & Wilkin, G.P. Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol. Cell. Neurosci. 16, 724–39 (2000).
Tortarolo, M. et al. Persistent activation of p38 mitogen-activated protein kinase in a mouse model of familial amyotrophic lateral sclerosis correlates with disease progression. Mol. Cell. Neurosci. 23, 180–192 (2003).
Drachman, D.B. et al. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann. Neurol. 52, 771–778 (2002).
Yoshihara, T. et al. Differential expression of inflammation- and apoptosis-related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 80, 158–167 (2002).
Marques, C.A. et al. Neurotoxic mechanisms caused by the Alzheimer's disease-linked Swedish amyloid precursor protein mutation: oxidative stress, caspases, and the JNK pathway. J. Biol. Chem. 278, 28294–28302 (2003).
Raoul, C. et al. Motoneuron death triggered by a specific pathway downstream of Fas. Potentiation by ALS-linked SOD1 mutations. Neuron 35, 1067–1083 (2002).
Saporito, M.S., Thomas, B.A. & Scott, R.W. MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J. Neurochem. 75, 1200–1208 (2000).
Saporito, M.S., Brown, E.M., Miller, M.S. & Carswell, S. CEP-1347/KT-7515, an inhibitor of c-Jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. J. Pharmacol. Exp. Ther. 288, 421–427 (1999).
Wang, W. et al. SP600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson's disease. Neurosci. Res. 48, 195–202 (2004).
Xia, X.G. et al. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 98, 10433–10438 (2001).
Trimmer, P.A., Smith, T.S., Jung, A.B. & Bennett, J.P., Jr. Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration 5, 233–239 (1996).
Emdadul Haque, M. et al. Apoptosis-inducing neurotoxicity of dopamine and its metabolites via reactive quinone generation in neuroblastoma cells. Biochim. Biophys. Acta 1619, 39–52 (2003).
Canals, S., Casarejos, M.J., de Bernardo, S., Rodriguez-Martin, E. & Mena, M.A. Glutathione depletion switches nitric oxide neurotrophic effects to cell death in midbrain cultures: implications for Parkinson's disease. J. Neurochem. 79, 1183–1195 (2001).
Stokes, A.H. et al. Dopamine toxicity in neuroblastoma cells: role of glutathione depletion by L-BSO and apoptosis. Brain Res. 858, 1–8 (2000).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301–1306 (2000).
German, D.C., Liang, C.L., Manaye, K.F., Lane, K. & Sonsalla, P.K. Pharmacological inactivation of the vesicular monoamine transporter can enhance 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration of midbrain dopaminergic neurons, but not locus coeruleus noradrenergic neurons. Neuroscience 101, 1063–1069 (2000).
Staal, R.G. & Sonsalla, P.K. Inhibition of brain vesicular monoamine transporter (VMAT2) enhances 1-methyl-4-phenylpyridinium neurotoxicity in vivo in rat striata. J. Pharmacol. Exp. Ther. 293, 336–342 (2000).
Gainetdinov, R.R. et al. Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. J. Neurochem. 70, 1973–1978 (1998).
Takahashi, N. & Uhl, G. Murine vesicular monoamine transporter 2: molecular cloning and genomic structure. Brain Res. Mol. Brain Res. 49, 7–14 (1997).
Spencer, J.P. et al. Conjugates of catecholamines with cysteine and GSH in Parkinson's disease: possible mechanisms of formation involving reactive oxygen species. J. Neurochem. 71, 2112–2122 (1998).
Berman, S.B. & Hastings, T.G. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J. Neurochem. 73, 1127–1137 (1999).
Dexter, D.T. et al. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann. Neurol. 35, 38–44 (1994).
Jha, N. et al. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson's disease. J. Biol. Chem. 275, 26096–26101 (2000).
Barker, J.E. et al. Depletion of brain glutathione results in a decrease of glutathione reductase activity; an enzyme susceptible to oxidative damage. Brain Res. 716, 118–122 (1996).
Mattiazzi, M. et al. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J. Biol. Chem. 277, 29626–29633 (2002).
Beretta, S. et al. Mitochondrial dysfunction due to mutant copper/zinc superoxide dismutase associated with amyotrophic lateral sclerosis is reversed by N-acetylcysteine. Neurobiol. Dis. 13, 213–221 (2003).
Khan, S.M. et al. Alzheimer's disease cybrids replicate β-amyloid abnormalities through cell death pathways. Ann. Neurol. 48, 148–155 (2000).
Ward, R.J. et al. Brain iron in the ferrocene-loaded rat: its chelation and influence on dopamine metabolism. Biochem. Pharmacol. 49, 1821–1826 (1995).
Connor, J.R. Iron acquisition and expression of iron regulatory proteins in the developing brain: manipulation by ethanol exposure, iron deprivation and cellular dysfunction. Dev. Neurosci. 16, 233–247 (1994).
Kaur, D. et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron 37, 899–909 (2003).
Cherny, R.A. et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30, 665–676 (2001).
Ritchie, C.W. et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol 60, 1685–1691 (2003).
Hottinger, A.F., Fine, E.G., Gurney, M.E., Zurn, A.D. & Aebischer, P. The copper chelator D-penicillamine delays onset of disease and extends survival in a transgenic mouse model of familial amyotrophic lateral sclerosis. Eur. J. Neurosci. 9, 1548–1551 (1997).
Wiedau-Pazos, M. et al. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science 271, 515–518 (1996).
Yim, M.B. et al. A gain-of-function of an amyotrophic lateral sclerosis–associated Cu,Zn-superoxide dismutase mutant: an enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc. Natl. Acad. Sci. USA 93, 5709–5714 (1996).
Cudkowicz, M.E. et al. Survival in transgenic ALS mice does not vary with CNS glutathione peroxidase activity. Neurology 59, 729–734 (2002).
Liu, R. et al. Increased mitochondrial antioxidative activity or decreased oxygen free radical propagation prevent mutant SOD1-mediated motor neuron cell death and increase amyotrophic lateral sclerosis-like transgenic mouse survival. J. Neurochem. 80, 488–500 (2002).
Lyons, T.J. et al. Mutations in copper-zinc superoxide dismutase that cause amyotrophic lateral sclerosis alter the zinc binding site and the redox behavior of the protein. Proc. Natl. Acad. Sci. USA 93, 12240–12244 (1996).
Estevez, A.G. et al. Induction of nitric oxide–dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science 286, 2498–2500 (1999).
Alexander, M.D. et al. “True” sporadic ALS associated with a novel SOD-1 mutation. Ann. Neurol. 52, 680–683 (2002).
Subramaniam, J.R. et al. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat. Neurosci. 5, 301–307 (2002).
Acknowledgements
This work was supported by the US National Institutes of Health. I apologize to my colleagues whose work was not discussed in detail or cited due to space limitations.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Andersen, J. Oxidative stress in neurodegeneration: cause or consequence?. Nat Med 10 (Suppl 7), S18–S25 (2004). https://doi.org/10.1038/nrn1434
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn1434
This article is cited by
-
27-Hydroxycholesterol activates the GSK-3β/β-catenin signaling pathway resulting in intestinal fibrosis by inducing oxidative stress: effect of dietary interventions
Inflammation Research (2024)
-
Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases
Translational Neurodegeneration (2023)
-
Developments for collagen hydrolysates as a multifunctional antioxidant in biomedical domains
Collagen and Leather (2023)
-
Modulation of cytotoxic amyloid fibrillation and mitochondrial damage of α-synuclein by catechols mediated conformational changes
Scientific Reports (2023)
-
Single-atom nanozymes towards central nervous system diseases
Nano Research (2023)