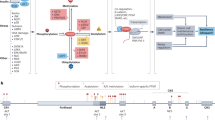
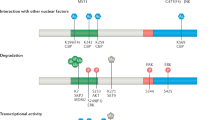
Key Points
-
Class O forkhead box transcription factors (FoxOs) have important roles in the regulation of lifespan and diseases such as diabetes and cancer.
-
The activity of many transcription factors is controlled by post-translational modifications, including phosphorylation, acetylation and ubiquitylation. In most cases, these modifications are reversible, thereby allowing dynamic regulation.
-
Phosphorylation of FoxOs can both inhibit (AKT/protein kinase B-mediated phosphorylation) and stimulate (c-Jun N-terminal kinase (JNK)-mediated phosphorylation) their transcriptional activity. Moreover, acetylation probably inhibits, whereas monoubiquitylation stimulates FoxO activity.
-
JNK-mediated phosphorylation, as well as acetylation and monoubiquitylation of FoxOs can be provoked by oxidative stress. These stress-induced modifications probably affect different facets of FoxO activity regulation, such as cellular localization, interaction with transcriptional cofactors and DNA binding.
-
FoxOs and p53 share cellular function — they both regulate cell-cycle progression and apoptosis when a cell encounters a stress that is possibly detrimental. They also share regulators of their activity: the transcriptional activity of both FoxO and p53 is controlled by the deacetylase SIRT1, the deubiquitylating enzyme USP7 and the serine–threonine kinase JNK. Importantly, SIRT1 and USP7 regulate the activity of FoxO and p53 in opposite directions, whereas JNK stimulates both proteins under stress conditions.
-
The current notion that there is a trade-off between lifespan and diseases such as diabetes and cancer can be explained at the molecular level. Lifespan extension by FoxO — for example, by enhanced activity of SIRT1 — is counterbalanced by an increased risk of getting cancer through inhibition of p53. Reversibly, USP7 decreases the risk of cancer by stimulating p53 but concomitantly inhibits FoxO, thereby decreasing lifespan. JNK lowers the risk of getting cancer through stimulation of p53 and enhances lifespan through stimulation of FoxO; however, JNK can also cause diabetes.
Abstract
Members of the class O of forkhead box transcription factors (FoxO) have important roles in metabolism, cellular proliferation, stress tolerance and probably lifespan. The activity of FoxOs is tightly regulated by post-translational modifications, including phosphorylation, acetylation and ubiquitylation. Several of the enzymes that regulate the turnover of these post-translational modifications are shared between FoxO and p53. These regulatory enzymes affect FoxO and p53 function in an opposite manner. This shared yet opposing regulatory network between FoxOs and p53 may underlie a 'trade-off' between disease and lifespan.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weigel, D., Jurgens, G., Kuttner, F., Seifert, E. & Jackle, H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell 57, 645–658 (1989).
Carlsson, P. & Mahlapuu, M. Forkhead transcription factors: key players in development and metabolism. Dev. Biol. 250, 1–23 (2002).
Furuyama, T., Nakazawa, T., Nakano, I. & Mori, N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem. J. 349, 629–634 (2000).
Kenyon, C., Chang, J., Gensch, E., Rudner, A. & Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464 (1993).
Lin, K., Dorman, J. B., Rodan, A. & Kenyon, C. DAF-16: an HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 278, 1319–1322 (1997).
Ogg, S. et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999 (1997). References 5 and 6 identified DAF-16 as a mediator of insulin signalling and lifespan.
Kops, G. J. et al. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398, 630–634 (1999).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Biggs, W. H., Meisenhelder, J., Hunter, T., Cavenee, W. K. & Arden, K. C. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl Acad. Sci. USA 96, 7421–7426 (1999).
Tang, E. D., Nunez, G., Barr, F. G. & Guan, K. L. Negative regulation of the forkhead transcription factor FKHR by Akt. J. Biol. Chem. 274, 16741–16746 (1999).
Takaishi, H. et al. Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc. Natl Acad. Sci. USA 96, 11836–11841 (1999).
Brownawell, A. M., Kops, G. J., Macara, I. G. & Burgering, B. M. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol. Cell Biol. 21, 3534–3546 (2001).
Burgering, B. M. & Kops, G. J. Cell cycle and death control: long live Forkheads. Trends Biochem. Sci. 27, 352–360 (2002).
Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956).
Barthel, A. et al. Differential regulation of endogenous glucose-6-phosphatase and phosphoenolpyruvate carboxykinase gene expression by the forkhead transcription factor FKHR in H4IIE-hepatoma cells. Biochem. Biophys. Res. Commun. 285, 897–902 (2001).
Nakae, J., Kitamura, T., Silver, D. L. & Accili, D. The forkhead transcription factor FOXO1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Invest. 108, 1359–1367 (2001).
Zhang, W. et al. FOXO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 281, 10105–10117 (2006).
Nemoto, S. & Finkel, T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295, 2450–2452 (2002).
Kops, G. J. et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419, 316–321 (2002). References 18 and 19 establish a role for FoxO in the control of oxidative stress in mammalian cells.
Dansen, T. B. et al. Regulation of sterol carrier protein gene expression by the forkhead transcription factor FOXO3a. J. Lipid Res. 45, 81–88 (2004).
Kops, G. J. et al. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol. Cell Biol. 22, 2025–2036 (2002).
Martinez-Gac, L., Marques, M., Garcia, Z., Campanero, M. R. & Carrera, A. C. Control of cyclin G2 mRNA expression by forkhead transcription factors: novel mechanism for cell cycle control by phosphoinositide 3-kinase and forkhead. Mol. Cell Biol. 24, 2181–2189 (2004).
Medema, R. H., Kops, G. J., Bos, J. L. & Burgering, B. M. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404, 782–787 (2000).
Schmidt, M. et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol. Cell Biol. 22, 7842–7852 (2002).
Puig, O. & Tjian, R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 19, 2435–2446 (2005).
Dijkers, P. F., Medema, R. H., Lammers, J. W., Koenderman, L. & Coffer, P. J. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10, 1201–1204 (2000).
Tang, T. T. et al. The forkhead transcription factor AFX activates apoptosis by induction of the BCL-6 transcriptional repressor. J. Biol. Chem. 277, 14255–14265 (2002).
Nakae, J. et al. Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nature Genet. 32, 245–253 (2002). Shows that aberrant FoxO regulation can cause diabetes in mice and thereby opposes normal insulin function.
Trotman, L. C. et al. Identification of a tumour suppressor network opposing nuclear Akt function. Nature 441, 523–527 (2006).
Paik, J. H. et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128, 309–323 (2007). Provides in vivo evidence for a role of FoxO as a tumour suppressor.
Pinkston, J. M., Garigan, D., Hansen, M. & Kenyon, C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science 313, 971–975 (2006).
Yilmaz, O. H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 (2006).
Tothova, Z. et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007). References 31–33 provide evidence for a role of PI3K signalling towards FoxO in the control of stem-cell fate.
Katic, M. & Kahn, C. R. The role of insulin and IGF-1 signaling in longevity. Cell. Mol. Life Sci. 62, 320–343 (2005).
Masoro, E. J. Caloric restriction and aging: an update. Exp. Gerontol. 35, 299–305 (2000).
Greer, E. L. & Brunet, A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24, 7410–7425 (2005).
Vogt, P. K., Jiang, H. & Aoki, M. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle 4, 908–913 (2005).
Lehtinen, M. K. et al. A conserved MST–FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125, 987–1001 (2006).
Graves, J. D. et al. Caspase-mediated activation and induction of apoptosis by the mammalian Ste20-like kinase Mst1. EMBO J. 17, 2224–2234 (1998).
Henderson, S. T. & Johnson, T. E. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 11, 1975–1980 (2001).
Brunet, A. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 (2004).
Essers, M. A. et al. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 23, 4802–4812 (2004).
Oh, S. W. et al. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc. Natl Acad. Sci. USA 102, 4494–4499 (2005).
Wang, M. C., Bohmann, D. & Jasper, H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell 121, 115–125 (2005).
Wang, M. C., Bohmann, D. & Jasper, H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev. Cell 5, 811–816 (2003). References 42–45 provide evidence that the lifespan of model organisms can be controlled through JNK-mediated stress signalling and JNK-mediated DAF-16 (FoxO) phosphorylation.
Sunayama, J., Tsuruta, F., Masuyama, N. & Gotoh, Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J. Cell Biol. 170, 295–304 (2005).
Huang, H., Regan, K. M., Lou, Z., Chen, J. & Tindall, D. J. CDK2-dependent phosphorylation of FOXO1 as an apoptotic response to DNA damage. Science 314, 294–297 (2006).
van der Horst, A. et al. FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2 (SIRT1). J. Biol. Chem. 279, 28873–28879 (2004).
Motta, M. C. et al. Mammalian SIRT1 represses forkhead transcription factors. Cell 116, 551–563 (2004).
Tissenbaum, H. A. & Guarente, L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410, 227–230 (2001). This study shows that sir-2 can extend lifespan in higher eukaryotes.
Daitoku, H. et al. Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc. Natl Acad. Sci. USA 101, 10042–10047 (2004).
Fukuoka, M. et al. Negative regulation of forkhead transcription factor AFX (Foxo4) by CBP-induced acetylation. Int. J. Mol. Med. 12, 503–508 (2003).
Kobayashi, Y. et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int. J. Mol. Med. 16, 237–243 (2005).
Mahmud, D. L. et al. Phosphorylation of forkhead transcription factors by erythropoietin and stem cell factor prevents acetylation and their interaction with coactivator p300 in erythroid progenitor cells. Oncogene 21, 1556–1562 (2002).
Perrot, V. & Rechler, M. M. The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription. Mol. Endocrinol. 19, 2283–2298 (2005).
Yang, Y., Hou, H., Haller, E. M., Nicosia, S. V. & Bai, W. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 24, 1021–1032 (2005).
Frescas, D., Valenti, L. & Accili, D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 280, 20589–20595 (2005).
Matsuzaki, H. et al. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc. Natl Acad. Sci. USA 102, 11278–11283 (2005).
Bordone, L. et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biol. 4, e31 (2005).
Lemieux, M. E. et al. The Sirt1 deacetylase modulates the insulin-like growth factor signaling pathway in mammals. Mech. Ageing Dev. 126, 1097–1105 (2005).
Berdichevsky, A., Viswanathan, M., Horvitz, H. R. & Guarente, L. C. elegans SIR-2. 1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell 125, 1165–1177 (2006).
Wang, Y. et al. C. elegans 14-3-3 proteins regulate life span and interact with SIR-2.1 and DAF-16/FOXO. Mech. Ageing Dev. 127, 741–747 (2006).
Gan, L. et al. FoxO-dependent and -independent mechanisms mediate SIRT1 effects on IGFBP-1 gene expression. Biochem. Biophys. Res. Commun. 337, 1092–1096 (2005).
Giannakou, M. E. & Partridge, L. The interaction between FOXO and SIRT1: tipping the balance towards survival. Trends Cell Biol. 14, 408–412 (2004). This review discusses the first results obtained on the regulation of FoxO by acetylation and SIRT1.
Nemoto, S., Fergusson, M. M. & Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 280, 16456–16460 (2005).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Bouras, T. et al. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J. Biol. Chem. 280, 10264–10276 (2005).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429, 771–776 (2004).
Huang, H. et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl Acad. Sci. USA 102, 1649–1654 (2005).
Matsuzaki, H., Daitoku, H., Hatta, M., Tanaka, K. & Fukamizu, A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc. Natl Acad. Sci. USA 100, 11285–11290 (2003).
Plas, D. R. & Thompson, C. B. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J. Biol. Chem. 278, 12361–12366 (2003).
Hu, M. C. et al. IκB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell 117, 225–237 (2004).
Pagano, M. et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269, 682–685 (1995).
Reich, N. C., Oren, M. & Levine, A. J. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol. Cell Biol. 3, 2143–2150 (1983).
Aoki, M., Jiang, H. & Vogt, P. K. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc. Natl Acad. Sci. USA 101, 13613–13617 (2004).
Mamillapalli, R. et al. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27KIP1 through the ubiquitin E3 ligase SCF (SKP2). Curr. Biol. 11, 263–267 (2001).
van der Horst, A. et al. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7/HAUSP. Nature Cell Biol. 8, 1064–1073 (2006). Shows for the first time that FOXOs are subject to monoubiquitylation and regulation by USP7 (HAUSP).
Essers, M. A. et al. Functional interaction between β-catenin and FOXO in oxidative stress signaling. Science 308, 1181–1184 (2005).
Tsuruta, F. et al. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 23, 1889–1899 (2004).
Aguirre, V., Uchida, T., Yenush, L., Davis, R. & White, M. F. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J. Biol. Chem. 275, 9047–9054 (2000).
el-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Nakano, K. & Vousden, K. H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 7, 683–694 (2001).
Yu, J., Zhang, L., Hwang, P. M., Kinzler, K. W. & Vogelstein, B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 7, 673–682 (2001).
Davies, M. J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 305, 761–770 (2003).
Trinei, M. et al. A p53–p66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene 21, 3872–3878 (2002).
Drane, P., Bravard, A., Bouvard, V. & May, E. Reciprocal down-regulation of p53 and SOD2 gene expression – implication in p53 mediated apoptosis. Oncogene 20, 430–439 (2001).
Pani, G. et al. Deregulated manganese superoxide dismutase expression and resistance to oxidative injury in p53-deficient cells. Cancer Res. 60, 4654–4660 (2000).
Hussain, S. P. et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 64, 2350–2356 (2004).
Zhao, Y. et al. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein–manganese superoxide dismutase. Cancer Res. 65, 3745–3750 (2005).
Kondoh, H. et al. Glycolytic enzymes can modulate cellular life span. Cancer Res. 65, 177–185 (2005).
Bensaad, K. et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126, 107–120 (2006).
Matoba, S. et al. p53 regulates mitochondrial respiration. Science 312, 1650–1653 (2006).
Zhou, S., Kachhap, S. & Singh, K. K. Mitochondrial impairment in p53-deficient human cancer cells. Mutagenesis 18, 287–292 (2003).
Maiyar, A. C., Huang, A. J., Phu, P. T., Cha, H. H. & Firestone, G. L. p53 stimulates promoter activity of the sgk. serum/glucocorticoid-inducible serine/threonine protein kinase gene in rodent mammary epithelial cells. J. Biol. Chem. 271, 12414–12422 (1996).
You, H. et al. p53-dependent inhibition of FKHRL1 in response to DNA damage through protein kinase SGK1. Proc. Natl Acad. Sci. USA 101, 14057–14062 (2004).
Stambolic, V. et al. Regulation of PTEN transcription by p53. Mol. Cell 8, 317–325 (2001).
Singh, B. et al. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 16, 984–993 (2002).
Fukazawa, T. et al. Differential involvement of the CD95 (Fas/APO-1) receptor/ligand system on apoptosis induced by the wild-type p53 gene transfer in human cancer cells. Oncogene 18, 2189–2199 (1999).
Seoane, J., Le, H. V., Shen, L., Anderson, S. A. & Massague, J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117, 211–223 (2004).
Tran, H. et al. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science 296, 530–534 (2002).
Kastan, M. B. et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71, 587–597 (1992).
Nemoto, S., Fergusson, M. M. & Finkel, T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 306, 2105–2108 (2004).
Adler, V. et al. Conformation-dependent phosphorylation of p53. Proc. Natl Acad. Sci. USA 94, 1686–1691 (1997).
Luo, J. et al. Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107, 137–148 (2001).
Vaziri, H. et al. hSIR2 (SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159 (2001). References 104 and 105 demonstrate a role for SIRT1 in the regulation of p53.
Li, M. et al. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 (2002). Identifies USP7 (HAUSP) as a deubiquitylating enzyme for p53.
Li, M. et al. Mono- versus polyubiquitination: differential control of p53 fate by Mdm2. Science 302, 1972–1975 (2003).
Buschmann, T. et al. Jun NH2-terminal kinase phosphorylation of p53 on Thr-81 is important for p53 stabilization and transcriptional activities in response to stress. Mol. Cell Biol. 21, 2743–2754 (2001).
Fuchs, S. Y., Adler, V., Pincus, M. R. & Ronai, Z. MEKK1/JNK signaling stabilizes and activates p53. Proc. Natl Acad. Sci. USA 95, 10541–10546 (1998).
Tyner, S. D. et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 415, 45–53 (2002).
Garcia-Cao, I. et al. 'Super p53' mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 21, 6225–6235 (2002). References 110 and 111 describe a role for p53 in the trade-off between cancer and lifespan.
van Heemst, D. et al. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp. Gerontol. 40, 11–15 (2005).
Ford, J., Jiang, M. & Milner, J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res. 65, 10457–10463 (2005).
Pruitt, K. et al. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet 2, e40 (2006).
Heltweg, B. et al. Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res. 66, 4368–4377 (2006).
Cheon, K. W. & Baek, K. H. HAUSP as a therapeutic target for hematopoietic tumors. Int. J. Oncol. 28, 1209–1215 (2006).
Masuya, D. et al. The HAUSP gene plays an important role in non-small cell lung carcinogenesis through p53-dependent pathways. J. Pathol. 208, 724–732 (2006).
Bennett, B. L., Satoh, Y. & Lewis, A. J. JNK: a new therapeutic target for diabetes. Curr. Opin. Pharmacol. 3, 420–425 (2003).
Waetzig, V. & Herdegen, T. Context-specific inhibition of JNKs: overcoming the dilemma of protection and damage. Trends Pharmacol. Sci. 26, 455–461 (2005).
Vlahopoulos, S. & Zoumpourlis, V. C. JNK: a key modulator of intracellular signaling. Biochemistry (Mosc.) 69, 844–854 (2004).
Wang, Y. & Tissenbaum, H. A. Overlapping and distinct functions for a Caenorhabditis elegans SIR2 and DAF-16/FOXO. Mech. Ageing Dev. 127, 48–56 (2006).
Demetrius, L. Aging in mouse and human systems: a comparative study. Ann. NY Acad. Sci. 1067, 66–82 (2006).
Masoro, E. J. Caloric restriction and aging: controversial issues. J. Gerontol. A Biol. Sci. Med. Sci. 61, 14–19 (2006).
Phelan, J. P. & Rose, M. R. Why dietary restriction substantially increases longevity in animal models but won't in humans. Ageing Res. Rev. 4, 339–350 (2005).
Dirks, A. J. & Leeuwenburgh, C. Caloric restriction in humans: potential pitfalls and health concerns. Mech. Ageing Dev. 127, 1–7 (2006).
Harman, D. The aging process. Proc. Natl Acad. Sci. USA 78, 7124–7128 (1981).
Asada, S. et al. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal. 19, 519–527 (2007).
Acknowledgements
We thank A. Brenkman, T. Dansen and L. Price for critical reading of the manuscript and members of our laboratory for comments and discussion. Work in the Burgering laboratory is supported by the Dutch Cancer Foundation (KWF) and the Netherlands Organization for Scientific Research (NWO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- 14-3-3 proteins
-
A family of ubiquitously expressed conserved regulatory molecules that can bind to functionally diverse signalling proteins, usually when they contain phosphorylated serine or threonine residues.
- Acetylation
-
Covalent binding of an acetyl moiety to the ε-amino groups of lysines and to the α-amino groups of N-terminal residues of proteins. Acetylation is catalysed by histone acetyltransferases (HATs) such as CBP, p300 and P/CAF.
- Ubiquitylation
-
Covalent binding of the small protein ubiquitin to lysine or N-terminal residues of proteins, either as a tag for their rapid cellular degradation or to alter, for example, cellular localization. Ubiquitylation is catalysed by a series of enzymes: E1, E2 and E3 ligases.
- Gluconeogenesis
-
The process of making glucose from its own breakdown products or from the breakdown products of lipids or proteins.
- Catalase
-
Haem-based enzyme that catalyses the breakdown of hydrogen peroxide into oxygen and water.
- Superoxide dismutase
-
(SOD). An enzyme that catalyses the dismutation of superoxide into oxygen and hydrogen peroxide, thereby functioning as an important antioxidant defence in nearly all cells that are exposed to oxygen. Manganese SOD (MnSOD) is localized in mitochondria.
- Sterol carrier protein
-
(SCP). SCPx functions as a thiolase and is involved in the breakdown of branched-chain fatty acids and in the biosynthesis of bile acids. SCP2 protects fatty acids from peroxidation.
- p27kip1
-
Along with p21cip1, this protein is a member of a family of cyclin-dependent kinase inhibitory proteins that counteract the activity of cyclins D and E on phosphorylation of the retinoblastoma protein and the subsequent cell-cycle progression through the G1–S transition.
- Insulin receptor substrate
-
(IRS). An IRS protein functions as a docking protein between the insulin receptor and a complex network of intracellular signalling molecules that contain Src-homology-2 domains. Four members (IRS1, IRS2, IRS3, IRS4) of this family have been identified.
- PTEN
-
Tumour-suppressor gene located on chromosome 10 that, when mutated, can result in an increased risk of cancer.
- Histone acetyltransferase
-
(HAT). An enzyme that acetylates core histones, which results in important regulatory effects on chromatin structure and assembly, and on gene transcription. Several HATs can also acetylate non-histone proteins.
- SIRT1
-
Trichostatin-A-insensitive Sir2-like class III histone deacetylases that can remove acetyl groups from substrates that include histone and non-histone proteins.
- Histone deacetylase
-
(HDAC). An enzyme that removes acetyl groups from histones but also from non-DNA-binding proteins. HDACs are sensitive to the deacetylase inhibitor trichostatin A.
- F-box protein
-
Protein that functions as the substrate recognition component of the so-called SCF (SKP1, cullin-1, F-box protein) complexes of E3 ubiquitin ligases.
- E3 ubiquitin ligase
-
An enzyme that attaches the molecular tag ubiquitin to proteins. Depending on the position and number of ubiquitin molecules that are attached, the ubiquitin tag can target proteins for degradation by the proteasome, sort them to specific subcellular compartments or modify their biological activity.
- Deubiquitylating enzyme
-
(DUB). An enzyme that removes ubiquitin moieties from substrate proteins, thereby changing the fate of that protein.
- BH3-only protein
-
A proapoptotic protein that mediates cell death in response to specific stimuli.
- Warburg effect
-
Causes cancer cells to rely preferentially on glycolysis for ATP production, unlike normal healthy cells.
- Type II diabetes
-
A form of diabetes, also known as 'adult-onset diabetes' or 'non-insulin-dependent diabetes', which usually affects people who are more than 40 years old. It results from adults body tissues becoming insensitive to insulin action.
Rights and permissions
About this article
Cite this article
van der Horst, A., Burgering, B. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol 8, 440–450 (2007). https://doi.org/10.1038/nrm2190
Issue Date:
DOI: https://doi.org/10.1038/nrm2190
This article is cited by
-
3D sheep rumen epithelial structures driven from single cells in vitro
Veterinary Research (2023)
-
From signalling pathways to targeted therapies: unravelling glioblastoma’s secrets and harnessing two decades of progress
Signal Transduction and Targeted Therapy (2023)
-
Molecular Hydrogen: an Emerging Therapeutic Medical Gas for Brain Disorders
Molecular Neurobiology (2023)
-
Acetylation of FOXO1 activates Bim expression involved in CVB3 induced cardiomyocyte apoptosis
Apoptosis (2023)
-
Expression Changes of SIRT1 and FOXO3a Significantly Correlate with Oxidative Stress Resistance Genes in AML Patients
Indian Journal of Hematology and Blood Transfusion (2023)