Key Points
-
At present there are approximately 200 antibodies in clinical trials, and the US Food and Drug Administration has approved several antibodies against cancer, transplant rejection, rheumatoid arthritis, Crohn's disease and antiviral prophylaxis. As the development of new therapeutic reagents into commercial products takes about 10 years, today's approved antibodies are generally mouse, chimeric or humanized antibodies, whereas the more recently developed reagents in clinical trials are typically complete human antibodies, of which some are derived from in vitro antibody libraries and transgenic mice.
-
The efficacy of antibodies in the prophylaxis and treatment of infectious diseases is likely to increase if a polyclonal human serum therapy is mimicked — that is, if a pool of highly specific and high-affinity monoclonal antibodies are administered.
-
In cancer therapy, the purpose of antibody administration is specific cancer-cell destruction or starving of tumours through taregting of the tumour vasculature. New technologies in the panning of antibody libraries on intact cells have made it possible to isolate antibodies to novel and promising cancer-specific and cancer-associated antigens.
-
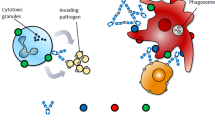
Together with cancer, inflammatory and autoimmune diseases are an important focus for antibody therapy. Antibodies have been developed to bind specific cytokines or their receptors for the purpose of inhibiting the detrimental effect of the cytokine. Inhibition of cytokines associated with inflammation and the modulation of the immune response by immune-cell depletion has been shown to be a viable therapy in autoimmune diseases.
-
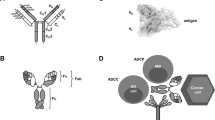
Small antibody fragments, such as single-chain Fv and Fab fragments, can do part of the job normally done by intact antibodies, such as blocking the action of a toxin or the ligand–receptor interaction, or inhibiting infection isuch as viral diseases. Antibody fragments can carry many different types of molecule to their targets as an effector moiety.
-
Bispecific antibodies have the ability to bind to two different antigens and have been used to cross-link various cells and molecules. Bispecific antibodies have primarily been used to been to re-direct effector cells to target cells.
-
A prerequisite for effective targeting is that antibodies should be able to penetrate tissues. Small antibody fragments are better than complete antibodies in this respect. However, small antibody fragments show rapid clearance from the circulation, and the fraction of the injected dose that reaches its target is at present too low, even for bivalent fragments. Therefore, manipulations to control antibody pharmacokinetics will be crucial for the success of therapeutic antibody fragments.
Abstract
Antibodies are highly specific, naturally evolved molecules that recognize and eliminate pathogenic and disease antigens. The past 30 years of antibody research have hinted at the promise of new versatile therapeutic agents to fight cancer, autoimmune diseases and infection. Technology development and the testing of new generations of antibody reagents have altered our view of how they might be used for prophylactic and therapeutic purposes. The therapeutic antibodies of today are genetically engineered molecules that are designed to ensure high specificity and functionality. Some antibodies are loaded with toxic modules, whereas others are designed to function naturally, depending on the therapeutic application. In this review, we discuss various aspects of antibodies that are relevant to their use as as therapeutic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Larrick, J. W. & Fry, K. E. Recombinant antibodies. Hum. Antibodies Hybridomas 2, 172–189 (1991).
Khazaeli, M. B., Conry, R. M. & LoBuglio, A. F. Human response to monoclonal antibodies. J. Immunother. 15, 42–52 (1994).
Morrison S. L., Johnson M. J., Herzenberg, L. A. & Oi, V. T. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc. Natl Acad. Sci. USA 21, 6851–6855 (1984).
Boulianne, G. L., Hozumi, N. & Shulman, M. J. Production of functional chimaeric mouse/human antibody. Nature 312, 643–646 (1984).
Bell, S. & Kamm, M. The clinical role of anti-TNFα antibody treatment in Crohn's disease. Aliment. Pharmacol. Ther. 14, 501–514 (2000).
Jones, P. T., Dear, P. H., Foote, J., Neuberger, M. S. & Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986).
Skerra, A. & Plückthun, A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science 240, 1038–1041 (1988).
Better, M., Chang, C. P., Robinson, R. R. & Horwitz, A. H. Escherichia coli secretion of an active chimeric antibody fragment. Science 240, 1041–1043 (1988).
McCafferty, J., Griffiths, A. D., Winter, G. & Chiswell, D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554 (1990).
Green, L. L. Antibody engineering via genetic engineering of the mouse: XenoMouse strains are a vehicle for the facile generation of therapeutic human monoclonal antibodies. J. Immunol. Methods 231, 11–23 (1999).
Cragg, M. S., French, R. R. & Glennie, M. J. Signalling antibodies in cancer therapy. Curr. Opin. Immunol. 11, 541–547 (1999).
Farah, R. A., Clinchy, B., Herrera, L. & Vitetta, E. S. The development of monoclonal antibodies for the therapy of cancer. Crit. Rev. Eukaryotic Gene Expr. 8, 321–356 (1998).
Berard, J. L. A review of interleukin-2 receptor antagonists in solid organ transplantations. Pharmacotherapy 19, 1127–1137 (1999).
Maini, R. et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concominant methotrexate: a randomised phase III trial. Lancet 254, 1932–1939 (1999).
Sandborn, W. J. & Hanauer, S. B. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results and safety. Inflamm. Bowel Dis. 5, 119–133 (1999).
Saez-Llorens, X. et al. Safety and pharmacokinetics of an intramuscular humanised antibody monoclonal antibody to respiratory syncitial virus in premature infants with bronchopulmonary dysplasia. Pediatr. Infect. Dis. 17, 787–791 (1998).
Huls, G. A. et al. A recombinant, fully human monoclonal antibody with antitumor activity constructed from phage-displayed antibody fragments. Nature Biotechnol. 17, 276–280 (1999).
Nagy, Z. A. et al. Fully human, HLA-DR-specific monoclonal antibodies efficiently induce programmed death of malignant lymphoid cells. Nature Med. 8, 801–807 (2002).
Mukherjee, J. et al. Production and characterization of protective human antibodies against shiga toxin 1. Infect. Immun. 70, 5896–5899 (2002).
Zeitlin, L., Cone, R. A., Moench, T. R. & Whaley K. J. Preventing infectious disease with passive immunization. Microbes Infect. 2, 701–708 (2000).
Lang, A. B., Cryz, S. J. Jr, Schurch, U., Ganss, M. T. & Bruderer, U. Immunotherapy with human monoclonal antibodies. Fragment A specificity of polyclonal and monoclonal antibodies is crucial for full protection against tetanus toxin. J. Immunol. 151, 466–472 (1993).
Maynard, J. A. et al. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nature Biotechnol. 20, 597–601 (2002).
Groothuis, J. R., Nishida, H. Prevention of respiratory syncytial virus infections in high-risk infants by monoclonal antibody (palivizumab). Pediatr. Int. 44, 235–241 (2002).
Parren, P. W. H. I. & Burton, D. R. The anti-viral activity of antibodies in vitro and in vivo. Adv. Immunol. 77, 195–262 (2001).
Burton, D. R. Antibodies, viruses and vaccines. Nature Rev. Immunol. 2, 706–713 (2002). An excellent review on the use of antibodies against viral diseases and the use of donor antibodies as tools for the development of recombinant vaccines, a process termed 'reverse vaccinology'.
Eren, R. et al. Clinical evaluation (phase I) of a combination of two human monoclonal antibodies to HBV: safety and antiviral properties. Hum. Antibodies 11, 27–28 (2002).
Jespers, L. S., Roberts, A., Mahler, S. M., Winter, G. & Hoogenboom, H. R. Guiding the selection of human antibodies from phage display repertoires to a single epitope of an antigen. Biotechnology 12, 899–903 (1994).
Haan, K. Product development: Rituxan's challenge in RA. Biocentury 10, A1–A5. (2002).
Looney, R. J. Treating human autoimmune disease by depleting B-cells. Ann. Rheum. Dis. 61, 863–866 (2002).
Kaplan, M. Eculizumab (Alexion). Curr. Opin. Investig. Drugs 3, 1017–1023 (2002).
Roovers, R. C., Van der Linden, E., De Bruine A. P., Arends, J. W. & Hoogenboom, H. R. Identification of colon tumour-associated antigens by phage antibody selction on primary colorectal carcinoma. Eur. J. Cancer 37, 542–549 (2001).
Larson, R. A. et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia 16, 1627–1636 (2002).
Epstein, A. L. et al. Two new monoclonal antibodies, Lym-1 and Lym-2, reactive human with B-lymphocyte and derived tumors with immunodiagnostic and immunotherapeutic potential. Cancer Res. 47, 830–840 (1987).
Gingrich, R. D., Dahle, C. E., Hoskins, K. F. & Senneff, M. J. Identification and characterization of a new surface membrane found predominantly on malignant B-lymphocytes. Blood 75, 2375–2387 (1990).
deNardo, S. J. et al. Treatment of B-cell malignancies with 131I Lym-1 monoclonal antibodies. Int. J. Cancer Suppl. 3, 96–101 (1988).
Clynes, R. A., Towers, T. L., Presta, L. G. and Ravetech, J. V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature Med. 6, 443–446 (2000).
Shields, R. L. et al. High resolution mapping of the binding site on human IgG1 for FcγRI, FcγRII, FcγRIII, and FcRn and design of IgG1 variants with improved binding to the FcγR. J. Biol. Chem. 276, 6591–6604 (2000).
Carter, P. Bispecific human IgG by design. J. Immunol. Methods 248, 7–15 (2001).
Dechant, M. & Valerius, T. IgA antibodies for therapy Crit. Rev. Oncol. Hematol. 39, 69–77 (2001).
Peipp, M. & Valerius, T. Bispecific antibodies targeting cancer cells Biochem. Soc. Trans. 30, 507–511 (2002).
Presta, L. G. Engineering antibodies for therapy. Curr. Pharm. Biotechnol. 3, 237–256 (2002). Whereas the focus for therapeutic antibodies has mostly been directed towards the antigen-binding part of antibodies, this paper describes the very important aspect of antibody effector functions and the modulation of the in vivo actions of antibodies.
Weir, A. N. C. et al. Formatting antibody fragments to mediate specific therapeutic functions. Biochem. Soc. Transactions 30, 512–516 (2002).
Choy, E. H. et al. Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology 41, 1133–1137 (2002).
Iliades, P., Kortt, A. A. & Hudson, P. J. Triabodies: single chain Fv fragments without a linker form trivalent trimers. FEBS Lett. 409, 437–441 (1997).
Kortt, A. A., Dolezal, O., Power, B. E. & Hudson, P. J. Dimeric and trimeric antibodies: high avidity scFvs for cancer targeting. Biomol. Eng 18, 95–108 (2001).
Holliger, P., Prospero, T. & Winter, G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc. Natl Acad. Sci. USA 90, 6444–6448 (1993). The pivotal paper describing diabodies. Since the publication of this paper, the concept has been used and developed by many groups and has become a means for generating bispecific antibodies.
Kontermann, R. E., Wing M. G. & Winter, G. Complement recruitment using bispecific diabodies. Nature Biotechnol. 15, 629–631 (1997).
Kipriyanov, S. M. et al. Synergistic antitumor effect of bispecific CD19 × CD3 and CD19 × CD16 diabodies in a preclinical model of non-Hodgkin's lymphoma. J. Immunol. 169, 137–144 (2002).
Holliger, P., Brissinck, J., Williams, R. L., Thielemans, K. & Winter, G. Specific killing of lymphoma cells by cytotoxic T-cells mediated by a bispecific diabody. Protein Eng. 9, 299–305 (1996).
Cochlovius, B. et al. Treatment of human B cell lymphoma xenografts with a CD3 × CD19 diabody and T cells. J. Immunol. 165, 888–895 (2000).
Kipriyanov, S. M. et al.Bispecific Tandem diabody for tumor therapy with improved antigen binding and pharmacokinetics. J. Mol. Biol. 293, 41–56 (1999).
Lunde, E. et al. Troybodies and Pepbodies. Biochem. Soc. Trans. 30, 500–506 (2002).
Adams, G. P. et al. High affinity restricts the localization and tumor penetration of single-chain fv antibody molecules. Cancer Res. 61, 4750–4755 (2001).
Nielsen, U. B., Adams, G. P., Weiner, L. M. & Marks, J. D. Targeting of bivalent anti-ErbB2 diabody antibody fragments to tumor cells is independent of the intrinsic antibody affinity. Cancer Res. 60, 6434–6440 (2000).
Schier, R. et al. Isolation of high-affinity monomeric human anti-c-erbB-2 single chain Fv using affinity-driven selection. J. Mol. Biol. 255, 28–43 (1996).
Hanes, J., Schaffitzel, C., Knappik, A. & Plückthun, A. Picomolar affinity antibodies from a fully synthetic naive library selected and evolved by ribosome display. Nature Biotechnol. 18, 1287–1292 (2000). Describes the selection of antibody fragments with the highest affinities reported so far. This also validates the ribosomal-display technology (see Box 2) as a potential tool for affinity maturation of antibodies.
Wörn, A. & Plückthun, A. Stability engineering of antibody single-chain Fv fragments. J. Mol. Biol. 305, 989–1010 (2001). A thorough review describing different aspects of protein stability of antibody fragments and the successful engineering applications of antibodies to increase their stability.
Padlan, E. A. A possible procedure for reducing the immunogenicity of antibody variable domains while preserving their ligand binding properties. Mol. Immunol. 28, 489–498 (1991).
Adair, F. Monoclonal antibodies: magic bullets or a shot in the dark. Drug Disc. World 3, 53–59 (2002).
Ghetie, V. & Ward, E. S. Multiple roles for the major histocompatibility complex class I- related receptor FcRn. Annu. Rev. Immunol. 18, 739–766 (2000).
Chapman, A. P. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv. Drug Deliv. Rev. 54, 531–545 (2002).
Anderson, W. L. & Tomasi T. B. Polymer modification of antibody to eliminate immune complex and Fc binding. J. Immunol. Methods 109, 37–42 (1988).
Suzuki, T., Kanbara, N., Tomono, T, Hayashi N. & Shinohara, I. Physiochemical and biological properties of poly (ethylene glycol)-coupled immunoglobulin G. Biochim. Biophys. Acta 788, 248–255 (1984).
Bona, C. A., Casares, S. & Brumeanu, T. D., Towards development of T cell vaccines. Immunol. Today 19, 126–133 (1998).
Lunde, E. et al. “Troy-bodies”: recombinant antibodies that target T cell epitopes to antigen presenting cells. Int. Rev. Immunol. 20, 647–673 (2001).
Lunde, E., Munthe, L. A., Vabo, A., Sandlie, I. & Bogen, B. Antibodies engineered with IgD specificity efficiently deliver integrated T-cell epitopes for antigen presentation by B cells. Nature Biotechnol. 17, 670–675 (1999).
Rasmussen, I. B., Lunde, E., Michaelsen, T. E., Bogen, B. & Sandlie, I. The principle of delivery of T cell epitopes to antigen-presenting cells applied to peptides from influenza virus, ovalbumin, and hen egg lysozyme: implications for peptide vaccination. Proc. Natl Acad. Sci. USA. 98, 10296–10301 (2001).
Dhodapkar, K. M., Krasovsky, J., Williamson, B. & Dhodapkar, M. V. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J. Exp. Med. 195, 125–133 (2002).
Irving, R. A., Coia G., Roberts, A., Nuttall, S. D. & Hudson, P. J. Ribosome display and affinity maturation: from antibodies to single V-domains and steps towards cancer therapeutics. J. Immunol. Methods. 248, 31–45 (2001).
Xu, L. et al. Directed evolution of high-affinity antibody mimics using mRNA display. Chem. Biol. 9, 933–942 (2002).
Fitzgerald, K. In vitro display technologies- new tools for drug discovery. Drug Discov. Today 5, 253–258 (2000).
Milstein, C. & Cuello, A. C. Hybrid hybridomas and their use in immunohistochemistry. Nature 305, 537–540 (1983).
Glennie, M. J., McBride, H. M., Worth A. T. & Stevenson, G. T. Preparation and performance of bispecific F(ab' γ)2 antibody containing thioether-linked Fab' γ fragments. J. Immunol. 139, 2367–2375 (1987).
Kettleborough, C. A., Saldanha, J., Heath VJ, Morrison, C. J. & Bendig, M. M. Humanization of a mouse monoclonal antibody by CDR-grafting: the importance of framework residues on loop conformation. Protein Eng. 4, 773–783 (1991).
Saldanha, J. W., Martin, A. C. & Leger, O. J. A single backmutation in the human kIV framework of a previously unsuccessfully humanized antibody restores the binding activity and increases the secretion in cos cells. Mol. Immunol. 36, 709–719 (1999).
de Wildt, R. M., Mundy, C. R., Gorick, B. D. & Tomlinson, I. M. Antibody arrays for high-throughput screening of antibody-antigen interactions. Nature Biotechnol. 18, 989–994 (2000). Describes a method for screening thousands of antibody-producing colonies. Further refinement and the use of this method will certainly be important in the antibody discovery field.
Das, R. C. & Morrow, K. J. Jr. Antibody Therapeutics: Production, Clinical Trials and Strategic Issues. (D&MD Publications, 2001).
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- IMMUNOGLOBULIN DOMAIN
-
Compactly folded globular units of approximately 110 amino acids that comprise immunoglobulin heavy and light chains.
- CYTOKINES
-
A class of small proteins released by one cell that affects the physiology of other cells locally and systemically in a particular fashion through binding to a specific receptor.
- EFFECTOR FUNCTIONS
-
The antigen-elimination processes mediated by immunoglobulins and initiated by the binding of effector molecules to the Fc part of the immunoglobulin. The common effector functions are complement-dependent cytotoxicity (CDC), phagocytosis and antibody-dependent cellular cytotoxicity (ADCC).
- COMPLEMENT-DEPENDENT CYTOTOXICITY
-
Once bound to antigen, both IgM and IgG can trigger a sequence of reactions by which serum proteins called complement factors are cleaved. One of the results is destruction of the target cell through complement-dependent cytotoxicity.
- PROGRAMMED CELL DEATH
-
Programmed cell death infers that cells are determined to die at a specific stage of development or having received a specific signal. The process is known as apoptosis. The cells shrivel and are engulfed by nearby phagocytic cells without eliciting any inflammatory response.
- HYBRIDOMA
-
An antibody-secreting B-cell line that is generated by fusing splenic-derived B cells with a plasmacytoma. A hybridoma produces the same antibody as the parent B cell and divides and grows in culture like the parent cancer cell. The antibody produced is monoclonal.
- SERUM THERAPY
-
The treatment of an infectious disease with the serum from an immunized animal or individual, and which contains antibody.
- POST-EXPOSURE PROPHYLAXIS
-
A treatment that is designed to protect an individual against a disease agent to which the individual has been recently exposed.
- PEGYLATION
-
The covalent attachment of the polymeric molecule poly-ethylene glycol (PEG) to proteins.
- TROYBODY
-
An antibody with specificity for an antigen-presenting cell and with an antigenic fragment inserted into a constant domain.
Rights and permissions
About this article
Cite this article
Brekke, O., Sandlie, I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat Rev Drug Discov 2, 52–62 (2003). https://doi.org/10.1038/nrd984
Issue Date:
DOI: https://doi.org/10.1038/nrd984
This article is cited by
-
sBioSITe enables sensitive identification of the cell surface proteome through direct enrichment of biotinylated peptides
Clinical Proteomics (2023)
-
Novel biomolecules in targeted cancer therapy: a new approach towards precision medicine
Medical Oncology (2023)
-
Co-optimization of therapeutic antibody affinity and specificity using machine learning models that generalize to novel mutational space
Nature Communications (2022)
-
Challenges and strategies for next-generation bispecific antibody-based antitumor therapeutics
Cellular & Molecular Immunology (2020)
-
Recent advances in therapeutic applications of neutralizing antibodies for virus infections: an overview
Immunologic Research (2020)