Key Points
-
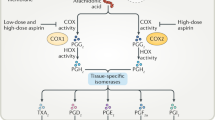
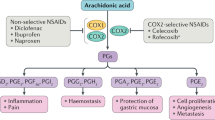
Aspirin remains one of the most commonly used drugs for the treatment of pain, fever and inflammation, and is increasingly used to reduce the risk of heart attacks and strokes.
-
A major drawback to the use of aspirin is its ability to cause ulcers in the stomach.
-
A new type of aspirin has been developed that consists of aspirin linked to a nitric oxide (NO)-releasing moiety — NO-aspirin.
-
In human and experimental studies, NO-aspirin does not cause damage in the stomach or intestine, in contrast to aspirin. However, NO-aspirin can still suppress inflammation and platelet aggregation.
-
NO-aspirin has been shown to have beneficial effects, often superseding those of aspirin, in experimental models of myocardial infarction (heart attack), cardiac ischaemia–reperfusion and coronary restenosis.
-
NO-aspirin has been shown to reduce blood pressure in experimental models of hypertension (high blood pressure), in contrast to conventional nonsteroidal anti-inflammatory drugs and selective cyclooxygenase-2 inhibitors.
-
NO-aspirin, unlike the parent drug, is able to potently suppress the production of several pro-inflammatory cytokines.
-
NO-aspirin produces its effects through various mechanisms, not all of which are related to its ability to inhibit cyclooxygenase activity and release nitric oxide.
-
NO-aspirin could represent an important advance in the prevention of serious cardiovascular events, such as heart attacks and stroke, being safer and more potent than aspirin.
Abstract
The use of low doses of aspirin on a daily basis has increased greatly in the past 20 years, based on observations that it can significantly reduce the risk of heart attacks and strokes. However, aspirin can also cause severe damage to the stomach. A modified version of aspirin that releases nitric oxide has been developed that seems to offer important advantages over its 103-year-old parent — namely, improved protection for the heart without the unwanted effects on the stomach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vane, J. R. The fight against rheumatism: from willow bark to COX-1 sparing drugs. J. Physiol. Pharmacol. 51, 573–586 (2000).
Patrono, C. Aspirin as an antiplatelet drug. N. Engl. J. Med. 330, 1287–1294 (1994).A comprehensive review of the rationale behind the use of aspirin to prevent cardiovascular disease, and its mechanisms of action.
Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. N. Engl. J. Med. 321, 129–135 (1989).
The RISC Group. Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. Lancet 336, 827–830 (1990).
The SALT Collaborative Group. Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. Lancet 338, 1345–1349 (1991).
Antiplatelet Trialist's Collaboration. Collaborative overview of randomized trials of antiplatelet therapy – I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 308, 81–106 (1994).
Roth, G. J., Stanford, N. & Majerus, P. W. Acetylation of prostaglandin synthase by aspirin. Proc. Natl Acad. Sci. USA 72, 3073–3076 (1975).
Patrono, C. et al. Low dose aspirin and inhibition of thromboxane B2 production in healthy subjects. Thromb. Res. 17, 317–327 (1980).
Gambino, M. C. et al. Selectivity of oral aspirin as an inhibitor of platelet vs. vascular cyclooxygenase activity is reduced by portocaval shunt in rats. J. Pharmacol. Exp. Ther. 245, 287–290 (1988).
Lee, M., Cryer, B. & Feldman, M. Dose effects of aspirin on gastric prostaglandins and stomach mucosal injury. Ann. Intern. Med. 120, 184–189 (1994).
Meade, T. W., Roderick, P. J., Brennan, P. J., Wilkes, H. C. & Kelleher, C. C. Extracranial bleeding and other symptoms due to low dose aspirin and low intensity oral anticoagulation. Thromb. Haemost. 68, 1–6 (1992).
Lanas, A. et al. Nitrovasodilators, low-dose aspirin, other nonsteroidal anti-inflammatory drugs, and the risk of upper gastrointestinal bleeding. N. Engl. J. Med. 343, 834–839 (2000).This epidemiological study shows that the use of even low doses of aspirin is associated with significant increases in the risk of GI bleeding. Moreover, the use of conventional NO donors can abolish the increased risk of GI bleeding that is associated with aspirin.
Wallace, J. L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology 112, 1000–1016 (1997).A review of the association between the use of nonsteroidal anti-inflammatory drugs, such as aspirin, and the adverse effects of those drugs on the digestive tract. This review includes a lengthy discussion of the various mechanisms through which drugs like aspirin cause damage in the stomach.
Soll, A. H., Weinstein, W. M., Kurata, J. & McCarthy, D. Nonsteroidal anti-inflammatory drugs and peptic ulcer disease. Ann. Intern. Med. 114, 307–319 (1991).
Bombardier, C. et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N. Engl. J. Med. 343, 1520–1528 (2000).This paper is significant for two main reasons. It is the first paper to show convincingly that selective COX-2 inhibitors cause less GI injury than a conventional anti-arthritis drug. However, it also shows that there is a significantly increased risk of serious cardiovascular problems with the selective COX-2 inhibitor.
Silverstein, F. E. et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. JAMA 284, 1247–1255 (2000).
Mukherjee, D., Nissen, S. E. & Topol, E. J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 286, 954–959 (2001).
Crofford, L. J. et al. Thrombosis in patients with connective tissue diseases treated with specific cyclooxygenase 2 inhibitors. A report of four cases. Arthritis Rheum. 43, 1891–1896 (2000).
Swan, S. K. et al. Effect of cyclooxygenase-2 inhibition on renal function in elderly persons receiving a low-salt diet. A randomized, controlled trial. Ann. Intern. Med. 133, 1–9 (2000).
Rossat, J., Maillard, M., Nussberger, J., Brunner, H. R. & Burnier, M. Renal effects of selective cyclooxygenase-2 inhibition in normotensive salt-depleted subjects. Clin. Pharmacol. Ther. 66, 76–84 (1999).
FDA Arthritis Advisory Committee Meeting. Review of NDA 21-042 Vioxx™ (rofecoxib) Merck Research Laboratories. [online] FDA Center for Drug Evaluation and Research (cited 26 Mar 02) 〈http://www.fda.gov/ohrms/dockets/ac/99/transcpt/3508t1.rtf〉 (1999).
Wallace, J. L. et al. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology 107, 173–179 (1994).
Wallace, J. L. et al. A diclofenac derivative without ulcerogenic properties. Eur. J. Pharmacol. 257, 249–255 (1994).
Wallace, J. L. et al. Gastrointestinal sparing anti-inflammatory drugs: the development of nitric oxide-relasing NSAIDs. Drug Dev. Res. 42, 144–149 (1997).
Bandarage, U. K. et al. Nitrosothiol esters of diclofenac: synthesis and pharmacological characterization as gastrointestinal-sparing prodrugs. J. Med. Chem. 43, 4005–4016 (2000).
MacNaughton, W. K., Cirino, G. & Wallace, J. L. Endothelium-derived relaxing factor (nitric oxide) has protective actions in the stomach. Life Sci. 45, 1869–1876 (1989).
Wallace, J. L. & Miller, M. J. S. Nitric oxide and mucosal defence: a little goes a long way. Gastroenterology 119, 512–520 (2000).
Davies, N. M. et al. NO-naproxen vs. naproxen: ulcerogenic, analgesic and anti-inflammatory effects. Aliment Pharmacol. Ther. 11, 69–79 (1997).
Wallace, J. L., McKnight, W., del Soldato, P., Baydoun, A. R. & Cirino, G. Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing aspirin derivative. J. Clin. Invest. 96, 2711–2718 (1995).This paper was the first to describe the actions of NO-aspirin; this drug was more potent than aspirin at blocking human platelet aggregation but, in the rat, did not cause any damage to the stomach (unlike aspirin).
Wallace, J. L. et al. In vivo antithrombotic effects of a nitric oxide-releasing aspirin derivative, NCX-4016. Thromb. Res. 93, 43–50 (1999).
Fiorucci, S. et al. IL-1β converting enzyme is a target for nitric oxide-releasing aspirin: new insights in the antiinflammatory mechanism of nitric oxide-releasing nonsteroidal anti-inflammatory drugs. J. Immunol. 165, 5245–5254 (2000).This was the first paper to illustrate that NO-aspirin could inhibit caspase activity, thereby reducing the synthesis of pro-inflammatory cytokines. So, NO-aspirin exerts anti-inflammatory effects that are distinct from those of aspirin.
Momi, S. et al. Prevention of pulmonary thromboembolism by NCX 4016, a nitric oxide-releasing aspirin. Eur. J. Pharmacol. 397, 177–185 (2000).
Carini, M., Aldini, G., Stefani, R., Orioli, M. & Facino, R. M. Nitrosylhemoglobin, an unequivocal index of nitric oxide release from nitroaspirin: in vitro and in vivo studies in the rat by ESR spectroscopy. J. Pharm. Biomed. Anal. 26, 509–518 (2001).
Muscara, M. N. et al. Vasorelaxant effects of a nitric oxide-releasing aspirin derivative in normotensive and hypertensive rats. Br. J. Pharmacol. 133, 1314–1322 (2001).
Yamamoto, T., Kakar, N. R., Vina, E. R., Johnson, P. E. & Bing, R. J. The effect of aspirin and two nitric oxide donors on the infarcted heart in situ. Life Sci. 67, 839–846 (2000).
Fulton, D. et al. Localization of endothelial nitric-oxide synthase phosphorylated on serine 1179 and nitric oxide in Golgi and plasma membrane defines the existence of two pools of active enzyme. J. Biol. Chem. 277, 4277–4284 (2002).
Takeuchi, K., Ukawa, H., Konaka, A., Kitamura, M. & Sugawa, Y. Effect of nitric oxide-releasing aspirin derivative on gastric functional and ulcerogenic responses in rats: comparison with plain aspirin. J. Pharmacol. Exp. Ther. 286, 115–121 (1998).
Fiorucci, S., Santucci, L., Mencarelli, A., Del Soldato, P. & Morelli, A. Gastrointestinal safety of a NO-releasing aspirin derivative (NCX-4016) in humans: a double blind placebo-controlled endoscopic study. Gastroenterology (in the press).
Minuz, P. et al. Antiaggregating and vasodilatory effects of a new nitroderivative of acetylsalicylic acid. Thromb. Res. 80, 367–376 (1995).
Fiorucci, S. et al. Gastrointestinal safety of nitric oxide-derived aspirin is related to inhibition of ICE-like cysteine proteases in rats. Gastroenterology 116, 1089–1106 (1999).
Wallace, J. L., McKnight, W., Wilson, T. L., Del Soldato, P. & Cirino, G. Reduction of shock-induced gastric damage by a nitric oxide-releasing aspirin derivative: role of neutrophils. Am. J. Physiol. 273, G1246–G1251 (1997).
Maricic, N. et al. Selective cyclo-oxygenase-2 inhibitors aggravate ischaemia–reperfusion injury in the rat stomach. Br. J. Pharmacol. 128, 1659–1666 (1999).
Klungel, O. H. et al. Antihypertensive drug therapies and the risk of ischemic stroke. Arch. Intern. Med. 161, 37–43 (2001).
Houston, M. C. Nonsteroidal anti-inflammatory drugs and anti-hypertensives. Am. J. Med. 90, 42S–47S (1991).
Whelton, A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am. J. Med. 106, 13S–24S (1999).
Muscará, M, N., et al. Selective cyclooxygenase-2 inhibition with celecoxib elevates blood pressure and promotes leukocyte adherence. Br. J. Pharmacol. 129, 1423–1430 (2000).
Muscará, M. N. et al. Anti-hypertensive properties of a nitric oxide-releasing naproxen derivative in 2-kidney, 1-clip rats. Am J Physiol Heart Circ Physiol 279, H528–H535 (2000).
Muscará, M. N., McKnight, W. & Wallace, J. L. Effect of a nitric oxide-releasing naproxen derivative on hypertension and gastric damage induced by chronic nitric oxide inhibition in the rat. Life Sci. 62, PL235–PL240 (1998).
Wallace, J. L. & Muscara, M. N. Selective COX-2 inhibitors: cardiovascular and gastrointestinal toxicity. Dig. Liver Dis. 33, S21–S28 (2001).
Ross, R. Atherosclerosis — an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Cooke, J. P. Is atherosclerosis an arginine deficiency disease? J. Invest. Med. 46, 377–380 (1998).
Drexler, H., Zeiher, A. M., Meinzer, K. & Just, H. Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet 338, 1546–1550 (1991).
Buga, G. M. et al. Arginase activity in endothelial cells: inhibition by NG-hydroxy-L-arginine during high-output NO production. Am. J. Physiol. 271, H1988–H1998 (1996).
Daghigh, F., Fukuto, J. M. & Ash, D. E. Inhibition of rat liver arginase by an intermediate in NO biosynthesis, NG-hydroxy-L-arginine: implications for the regulation of nitric oxide biosynthesis by arginase. Biochem. Biophys. Res. Commun. 202, 174–180 (1994).
Buga, G. M., Wei, L., Bauer, P. M., Fukoto, J. M. & Ignarro, L. J. NG-hydroxy-L-arginine and nitric oxide inhibit Caco-2 tumor cell proliferation by distinct mechanisms. Am. J. Physiol. 275, R1256–R1264 (1998).
Blachier, F., Robert, V., Selamnia, M., Mayeur, C. & Duee, P. H. Sodium nitroprusside inhibits proliferation and putrescine synthesis in human colon carcinoma cells. FEBS Lett. 396, 315–318 (1996).
Bauer, P. M., Fukuto, J. M., Buga, G. M., Pegg, A. E. & Ignarro, L. J. Nitric oxide inhibits ornithine decarboxylase by S-nitrosylation. Biochem. Biophys. Res. Commun. 262, 355–358 (1999).
Ignarro, L. J. et al. Role of the arginine–nitric oxide pathway in the regulation of vascular smooth muscle cell proliferaton. Proc. Natl Acad. Sci. USA 98, 4202–4208 (2001).This paper provides several lines of evidence to support the hypothesis that nitric oxide modulates vascular-smooth-muscle proliferation through effects on ornithine decarboxylase and arginase.
Liu, M. W., Roubin, G. S. & King, S. B. Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation 79, 1374–1387 (1989).
Schwartz, R. S., Holmes, D. R. & Topol, E. J. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J. Am. Coll. Cardiol. 20, 1284–1293 (1992).
Folts, J. D., Schafer, A. I., Loscalzo, J., Willerson, J. T. & Muller, J. E. A perspective on the potential problems with aspirin as an antithrombotic agent: a comparison of studies in an animal model with clinical trials. J. Am. Coll. Cardiol. 33, 295–303 (1999).
Awtry, E. H., Loscalzo, J. Aspirin. Circulation 101, 1206–1218 (2000).
Myers, P. R. et al. Restenosis is associated with decreased coronary artery nitric oxide synthase. Int. J. Cardiol. 55, 183–191 (1996).
Schwarzacher, S. P. et al. Local intramural delivery of L-arginine enhances nitric oxide generation and inhibits lesion formation after balloon angioplasty. Circulation 95, 1863–1869 (1997).
Niebauer, J. et al. Local L-arginine delivery after balloon angioplasty reduces monocyte binding and induces apoptosis. Circulation 100, 1830–1835 (1999).
Le Tourneau, T. et al. Role of nitric oxide in restenosis after experimental balloon angioplasty in the hypercholesterolemic rabbit: effects on neointimal hyperplasia and vascular remodeling. J. Am. Coll. Cardiol. 33, 876–882 (1999).
Napoli, C. et al. Effects of nitric oxide-releasing aspirin versus aspirin on restenosis in hypercholesterolemic mice. Proc. Natl Acad. Sci. USA 98, 2860–2864 (2001).Using a mouse model, these investigators showed the superiority of NCX4016 over aspirin in preventing the process of restenosis.
Napoli, C. et al. Efficacy and age-related effects of nitric oxide-releasing aspirin on experimental restenosis. Proc. Natl Acad. Sci. USA 99, 1689–1694 (2002).
Asako, H., Kubes, P., Wallace, J. L., Wolf, R. E. & Granger, D. N. Modulation of leukocyte adhesion in rat mesenteric venules by aspirin and salicylate. Gastroenterology 103, 146–152 (1992).
Wallace, J. L. et al. Enhanced anti-inflammatory effects of a nitric oxide-releasing derivative of mesalamine in rats. Gastroenterology 117, 557–566 (1999).
Gaboury, J., Woodman, R. C., Granger, D. N., Reinhardt, P. & Kubes, P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am. J. Physiol. 265, H862–H867 (1993).
Armstead, V. E. et al. Regulation of P-selectin expression in human endothelial cells by nitric oxide. Am. J. Physiol. 273, H740–H746 (1997).
Mitchell, D. J., Yu, J. & Tyml, K. Local L-NAME decreases blood flow and increases leukocyte adhesion via CD18. Am. J. Physiol. 274, H1264–H1268 (1998).
Wallace, J. L., Cirino, G., McKnight, G. W. & Elliott, S. N. Reduction of gastrointestinal injury in acute endotoxic shock by flurbiprofen nitroxybutylester. Eur. J. Pharmacol. 280, 63–68 (1995).
Rossoni, G., Berti, M., de Gennaro Colonna, V., Del Soldato, P. & Berti, F. Myocardial protection by the nitroderivative of aspirin, NCX 4016: in vitro and in vivo experiments in the rabbit. Ital. Heart J. 1, 146–155 (2000).
Rossoni, G., Muscara, M. N., Cirino, G. & Wallace, J. L. Inhibition of cyclooxygenase-2 exacerbates ischaemia-induced acute myocardial dysfunction in the rabbit. Br. J. Pharmacol. (in the press).
Berti, F. et al. Nitric oxide and prostacyclin influence coronary vasomotor tone in perfused rabbit heart and modulate endothelin-1 activity. J. Cardiovasc. Pharmacol. 22, 321–326 (1993).
Schrör, K., Zimmermann, K. C. & Tannhäuser, R. Augmented myocardial ischaemia by nicotine: mechanisms and their possible significance. Br. J. Pharmacol. 125, 79–86 (1998).
Miyauchi, T. et al. Increased plasma concentrations of endothelin-1 and big endothelin-1 in acute myocardial infarction. Lancet 2, 53–54 (1989).
Rossoni, G., Manfredi, B., de Gennaro Colonna, V., Bernareggi, M. & Berti, F. The nitroderivative of aspirin, NCX 4016, reduces infarct size caused by myocardial ischemia-reperfusion in the anesthetized rat. J. Pharmacol. Exp. Ther. 297, 380–387 (2001).
Fiorucci, S., Antonelli, E., Burgaud, J. L. & Morelli, A. NO-releasing NSAIDs: a review of their current status. Drug Saf. 24, 801–811 (2001).
Al-Swayeh, O. A., Clifford, R. H., Del Soldato, P. & Moore, P. K. A comparison of the anti-inflammatory and anti-nociceptive activity of nitroaspirin and aspirin. Br. J. Pharmacol. 129, 343–350 (2000).
Wallace, J. L., Reuter, B. K. & Cirino, G. Nitric oxide-releasing non-steroidal anti-inflammatory drugs: a novel approach for reducing gastrointestinal toxicity. J. Gastroenterol. Hepatol. 9 (Suppl. 1), S40–S44 (1994).
Salvesen, G. S. & Dixit, V. M. Caspases: intracellular signalling by proteolysis. Cell 91, 443–446 (1997).
Nicholson, D. W. From bench to clinic with apoptosis-based therapeutic agents. Nature 407, 810–816 (2000).
Reed, J. C. Apoptosis-based therapies. Nature Rev. Drug Discovery 1, 111–121 (2002).
Dinarello, C. A. & Margolis, N. H. Stopping the cuts: the recently discovered enzymes that process the precursors of inflammatory cytokines are good targets for the design of new anti-inflammatory therapeutic agents. Curr. Biol. 5, 587–590 (1995).
Thonberry, N. & Lazebnick, Y. Caspases: enemies within. Science 281, 1312–1316 (1998).
Stamler, J. S., Lamas, S. & Fang, F. C. Nitrosylation. The prototypic redox-based signaling mechanism. Cell 106, 675–683 (2001).
Dimmeler, S., Haendeler, J., Nehls, M. & Zeiher, A. M. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1 converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J. Exp. Med. 185, 601–607 (1997).
Sims, J. E. IL-1 and IL-18 receptors, and their extended family. Curr. Opin. Immunol. 14, 117–122 (2002).
Tsutsui, H. et al. Caspase-1-independent, Fas/Fas ligand-mediated IL-18 secretion from macrophages causes acute liver injury in mice. Immunity 11, 359–367 (1999).
Fantuzzi, G., Reed, D. A. & Dinarello, C. A. IL-12-induced IFN-γ is dependent on caspase-1 processing of the IL-18 precursor. J. Clin. Invest. 104, 761–767 (1999).
Gu, Y. et al. Activation of interferon-γ inducing factor is mediated by interleukin-1β converting enzyme. Science 275, 206–209 (1997).
Tsutsui, H. et al. IL-18 accounts for both TNF-α- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J. Immunol. 159, 3961–3967 (1997).
Kim, Y. M., Talanian, R. V., Li, J. & Billiar, T. R. Nitric oxide prevents IL-1β and IFNγ-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1β converting enzyme). J. Immunol. 161, 4122–4128 (1998).
Fiorucci, S. et al. IL-1β converting enzyme is a target for nitric oxide-releasing aspirin: new insights in the antiinflammatory mechanism of nitric oxide-releasing nonsteroidal antiinflammatory drugs. J. Immunol. 165, 5245–5254 (2000).
Fiorucci, S. NO-releasing NSAIDs are caspase inhibitors. Trends Immunol. 22, 232–235 (2001).A review of the evidence that indicates that a principal mechanism of action of NO-aspirin in reducing inflammation is by means of S-nitrosylation of the caspase family of enzymes.
Fiorucci, S. et al. NO-aspirin protects from T-cell mediated liver injury by inhibiting caspase-dependent processing of TH1-like cytokines. Gastroenterology 118, 404–421 (2000).
Hauss-Wegrzyniak, B., Vraniak, P. & Wenk, G. L. The effects of a novel NSAID on chronic neuroinflammation are age dependent. Neurobiol. Aging 20, 305–313 (1999).
Jantzen, P. T. et al. Microglial activation and β-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-Inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J. Neurosci. 22, 2246–2254 (2002).
Clementi, E., Brown, G. C., Foxwell, N. & Moncada, S. On the mechanism by which vascular endothelial cells regulate their oxygen consumption. Proc. Natl Acad. Sci. USA 96, 1559–1562 (1999).
Cines, D. B. et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91, 3527–3561 (1998).
Paxinou, E. et al. Dynamic regulation of metabolism and respiration by endogenously produced nitric oxide protects against oxidative stress. Proc. Natl Acad. Sci. USA 98, 11575–11580 (2001).
Fiorucci, S. et al. Nitric oxide-releasing NSAIDs inhibit interleukin-1β converting enzyme-like cysteine proteases and protect endothelial cells from apoptosis induced by TNFα. Aliment. Pharmacol. Ther. 13, 421–435 (1999).
Acknowledgements
J.L.W. is an Alberta Heritage Foundation for Medical Research Senior Scientist.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Cancer.gov
LocusLink
Medscape DrugInfo
OMIM
WormBase
Glossary
- PROPHYLAXIS
-
Preventative treatment against a disease.
- ISCHAEMIA–REPERFUSION
-
A local reduction of blood flow, usually due to an obstruction, which is followed by a restoration of blood flow.
- NORMOTENSIVE
-
A state of normal blood pressure.
- VASOCONSTRICTOR
-
A substance that causes a narrowing of blood vessels, such that there is a slowing of the flow of blood.
- ATHEROSCLEROSIS
-
Hardening of the arteries, which is caused by irregularly distributed fat deposits on the inner lining of these blood vessels (particularly in medium and large arteries).
- ATHEROGENESIS
-
The formation of fat deposits on the inner lining of blood vessels.
- PERCUTANEOUS TRANSLUMINAL ANGIOPLASTY
-
An operation for enlarging a narrowed artery by introducing, through the skin, a balloon-tipped catheter into the artery, and dilating the lumen of the artery on withdrawal of the inflated catheter tip.
- RESTENOSIS
-
Recurrence of a narrowing of a blood vessel after corrective surgery or angioplasty has been performed.
- NEOINTIMA
-
A newly formed inner lining of a blood vessel (such as that which is formed after angioplasty has been performed).
- HYPERPLASIA
-
An increase in the number of cells in a tissue, such that the bulk of the tissue is increased.
- ANGIOPLASTY
-
A procedure to reconstruct a blood vessel (often referring to the physical removal of atherosclerotic plaques (fat deposits) from the inner lining of blood vessels).
- MESENTERIC VENULES
-
Small veins in the abdomen that carry blood from the digestive tract to the portal vein, and, through that vessel, to the liver.
- PROSTACYCLIN
-
A chemical that is produced by various cells, including those on the inner lining of blood vessels, which can cause a widening of blood vessels (thereby increasing the flow of blood) and prevent platelets from sticking to one another.
- ENDOPROTEASE
-
An enzyme that digests proteins by cleaving peptide bonds (between neighbouring amino acids) other than those at either end of the protein.
Rights and permissions
About this article
Cite this article
Wallace, J., Ignarro, L. & Fiorucci, S. Potential cardioprotective actions of no-releasing aspirin. Nat Rev Drug Discov 1, 375–382 (2002). https://doi.org/10.1038/nrd794
Issue Date:
DOI: https://doi.org/10.1038/nrd794
This article is cited by
-
1,5-Diarylpyrroles as potent antitubercular and anti-inflammatory agents
Chemistry of Heterocyclic Compounds (2017)
-
Strategies to increase nitric oxide signalling in cardiovascular disease
Nature Reviews Drug Discovery (2015)
-
Bile acid activated receptors are targets for regulation of integrity of gastrointestinal mucosa
Journal of Gastroenterology (2015)
-
Bioengineered Colorectal Cancer Drugs: Orally Delivered Anti-Inflammatory Agents
Cell Biochemistry and Biophysics (2015)
-
Design, synthesis and biological evaluation of 4-(1-(4(sulphanilamide)phenyl)-3-(methyl)-1H-pyrazol-5-yl)dine urea and N-acyl derivatives as a soluble epoxide hydrolase inhibitors
Medicinal Chemistry Research (2014)