Key Points
-
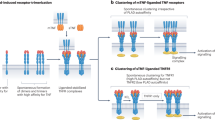
Inhibitors of tumour necrosis factor (TNF) are among the most successful protein-based drugs for reducing inflammation associated with several autoimmune diseases. Five distinct antibody- or receptor-based drugs directed at blocking TNF are approved for treating rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis, psoriasis, ankylosing spondylitis, Crohn's disease and ulcerative colitis.
-
TNF belongs to a superfamily of molecules comprising 19 structurally related proteins (ligands) that bind to one or more molecules in a family of 29 structurally similar receptors. Members of this superfamily initiate a broad range of physiological functions and provide key communication to regulate the development and activity of the immune, nervous, bone and ectodermal systems in mammals.
-
Biologics targeting many of the proteins related to TNF or their cognate receptors are now in preclinical or clinical development.
-
Therapeutic approaches include generating blocking and/or neutralizing reagents to prevent the TNFSF–TNFRSF interactions in order to reduce pathology in autoimmune and inflammatory diseases or other indications; developing agonist reagents to enhance signalling through a TNFRSF member to augment immune responses against tumours; and producing immunotoxins or death-inducing reagents that bind to TNFSF or TNFRSF members for the direct killing of tumours.
-
Studies so far have led to the successful development of three drugs in addition to the TNF inhibitors: targeting CD30 to kill certain cancers, blocking receptor activator of NF-κB ligand (RANKL) to reduce osteoporosis, and inhibiting B cell activating factor (BAFF) to suppress symptoms of systemic lupus erythematosus.
-
This article reviews the current range of biologics targeting all of the molecules in the TNF and TNF receptor superfamily that have been or are in clinical trials for autoimmune and inflammatory diseases, cancer and several other indications, focusing on the challenges in their development as well as emerging therapeutic targets.
Abstract
Inhibitors of tumour necrosis factor (TNF) are among the most successful protein-based drugs (biologics) and have proven to be clinically efficacious at reducing inflammation associated with several autoimmune diseases. As a result, attention is focusing on the therapeutic potential of additional members of the TNF superfamily of structurally related cytokines. Many of these TNF-related cytokines or their cognate receptors are now in preclinical or clinical development as possible targets for modulating inflammatory diseases and cancer as well as other indications. This Review focuses on the biologics that are currently in clinical trials for immune-related diseases and other syndromes, discusses the successes and failures to date as well as the expanding therapeutic potential of modulating the activity of this superfamily of molecules.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bodmer, J. L., Schneider, P. & Tschopp, J. The molecular architecture of the TNF superfamily. Trends Biochem. Sci. 27, 19–26 (2002).
Locksley, R. M., Killeen, N. & Lenardo, M. J. The TNF & TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
Croft, M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nature Rev. Immunol. 3, 609–620 (2003).
Ware, C. F. Network communications: lymphotoxins, LIGHT, and TNF. Annu. Rev. Immunol. 23, 787–819 (2005).
Croft, M. The role of TNF superfamily members in T-cell function and diseases. Nature Rev. Immunol. 9, 271–285 (2009). This is an in-depth focus on the basic biology of several TNFR molecules that control T cell activity.
Steinberg, M. W., Cheung, T. C. & Ware, C. F. The signaling networks of the herpesvirus entry mediator (TNFRSF14) in immune regulation. Immunol. Rev. 244, 169–187 (2011).
Croft, M. et al. TNF superfamily in inflammatory disease: translating basic insights. Trends Immunol. 33, 144–152 (2012).
Mackay, F. & Schneider, P. Cracking the BAFF code. Nature Rev. Immunol. 9, 491–502 (2009).
Stohl, W. & Hilbert, D. M. The discovery and development of belimumab: the anti-BLyS-lupus connection. Nature Biotech. 30, 69–77 (2012). This review provides a historic backdrop to the development of drugs targeting BAFF.
Treml, J. F., Hao, Y., Stadanlick, J. E. & Cancro, M. P. The BLyS family: toward a molecular understanding of B cell homeostasis. Cell Biochem. Biophys. 53, 1–16 (2009).
Benson, M. J. et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 180, 3655–3659 (2008).
Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 63, 3918–3930 (2011).
Navarra, S. V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 377, 721–731 (2011).
Dall'Era, M. et al. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 56, 4142–4150 (2007).
Munafo, A., Priestley, A., Nestorov, I., Visich, J. & Rogge, M. Safety, pharmacokinetics and pharmacodynamics of atacicept in healthy volunteers. Eur. J. Clin. Pharmacol. 63, 647–656 (2007).
Nestorov, I., Munafo, A., Papasouliotis, O. & Visich, J. Pharmacokinetics and biological activity of atacicept in patients with rheumatoid arthritis. J. Clin. Pharmacol. 48, 406–417 (2008).
Genovese, M. C., Kinnman, N., de La Bourdonnaye, G., Pena Rossi, C. & Tak, P. P. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis Rheum. 63, 1793–1803 (2011).
van Vollenhoven, R. F., Kinnman, N., Vincent, E., Wax, S. & Bathon, J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 63, 1782–1792 (2011).
Hartung, H. P. & Kieseier, B. C. Atacicept: targeting B cells in multiple sclerosis. Ther. Adv. Neurol. Disord. 3, 205–216 (2010).
Kim, S. S., Richman, D. P., Zamvil, S. S. & Agius, M. A. Accelerated central nervous system autoimmunity in BAFF-receptor-deficient mice. J. Neurol. Sci. 306, 9–15 (2011).
Matsushita, T., Yanaba, K., Bouaziz, J. D., Fujimoto, M. & Tedder, T. F. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 118, 3420–3430 (2008).
Yang, M. et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J. Immunol. 184, 3321–3325 (2010).
Huntington, N. D. et al. A BAFF antagonist suppresses experimental autoimmune encephalomyelitis by targeting cell-mediated and humoral immune responses. Int. Immunol. 18, 1473–1485 (2006).
Zhou, X. et al. BAFF promotes Th17 cells and aggravates experimental autoimmune encephalomyelitis. PLoS ONE 6, e23629 (2011).
Lin, W. Y. et al. Anti-BR3 antibodies: a new class of B-cell immunotherapy combining cellular depletion and survival blockade. Blood 110, 3959–3967 (2007).
Seshasayee, D. et al. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity 18, 279–288 (2003).
Varfolomeev, E. et al. APRIL-deficient mice have normal immune system development. Mol. Cell. Biol. 24, 997–1006 (2004).
Castigli, E. et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nature Genet. 37, 829–834 (2005).
Xiao, Y., Motomura, S. & Podack, E. R. APRIL (TNFSF13) regulates collagen-induced arthritis, IL-17 production and Th2 response. Eur. J. Immunol. 38, 3450–3458 (2008).
Jacob, C. O. et al. Dispensability of APRIL to the development of systemic lupus erythematosus in NZM 2328 mice. Arthritis Rheum. 64, 1610–1619 (2012).
Jiang, C., Loo, W. M., Greenley, E. J., Tung, K. S. & Erickson, L. D. B cell maturation antigen deficiency exacerbates lymphoproliferation and autoimmunity in murine lupus. J. Immunol. 186, 6136–6147 (2011).
Peters, A. L., Stunz, L. L. & Bishop, G. A. CD40 and autoimmunity: the dark side of a great activator. Semin. Immunol. 21, 293–300 (2009).
Law, C. L. & Grewal, I. S. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv. Exp. Med. Biol. 647, 8–36 (2009). References 32 and 33 provide a strong insight into the immunostimulatory activities of CD40.
Boumpas, D. T. et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 48, 719–727 (2003).
Patel, V. L., Schwartz, J. & Bussel, J. B. The effect of anti-CD40 ligand in immune thrombocytopenic purpura. Br. J. Haematol. 141, 545–548 (2008).
Kawai, T., Andrews, D., Colvin, R. B., Sachs, D. H. & Cosimi, A. B. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nature Med. 6, 114 (2000). This work provides an example of an unexpected outcome of therapy due to an unappreciated expression pattern of CD40L. Expression databases may avoid this problem in future trials.
Schuler, W. et al. Efficacy and safety of ABI793, a novel human anti-human CD154 monoclonal antibody, in cynomolgus monkey renal allotransplantation. Transplantation 77, 717–726 (2004).
Davis, J. C. et al. Phase I clinical trial of a monoclonal antibody against CD40-ligand (IDEC-131) in patients with systemic lupus erythematosus. J. Rheumatol. 28, 95–101 (2001).
Kalunian, K. C. et al. Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 46, 3251–3258 (2002).
Adams, A. B. et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J. Immunol. 174, 542–550 (2005).
Thompson, P. et al. CD40-specific costimulation blockade enhances neonatal porcine islet survival in nonhuman primates. Am. J. Transplant. 11, 947–957 (2011).
Haanstra, K. G. et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation 75, 637–643 (2003).
't Hart, B. A. et al. Treatment with chimeric anti-human CD40 antibody suppresses MRI-detectable inflammation and enlargement of pre-existing brain lesions in common marmosets affected by MOG-induced EAE. J. Neuroimmunol. 163, 31–39 (2005).
Kasran, A. et al. Safety and tolerability of antagonist anti-human CD40 Mab ch5D12 in patients with moderate to severe Crohn's disease. Aliment. Pharmacol. Ther. 22, 111–122 (2005).
Aoyagi, T. et al. A human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys: induction and maintenance therapy. Am. J. Transplant. 9, 1732–1741 (2009).
Oura, T. et al. Long-term hepatic allograft acceptance based on CD40 blockade by ASKP1240 in nonhuman primates. Am. J. Transplant. 12, 1740–1754 (2012).
Ware, C. F. Targeting the LIGHT-HVEM pathway. Adv. Exp. Med. Biol. 647, 146–155 (2009).
Browning, J. L. Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol. Rev. 223, 202–220 (2008). This article provides an excellent overview of translation efforts examining the lymphotoxin system as a target in autoimmune disease.
Chiang, E. Y. et al. In vivo depletion of lymphotoxin-α expressing lymphocytes inhibits xenogeneic graft-versus-host-disease. PLoS ONE 7, e33106 (2012).
Emu, B. et al. Safety, pharmacokinetics, and biologic activity of pateclizumab, a novel monoclonal antibody targeting lymphotoxin α: results of a phase I randomized, placebo-controlled trial. Arthritis Res. Ther. 14, R6 (2012).
Chiang, E. Y. et al. Targeted depletion of lymphotoxin-α-expressing TH1 and TH17 cells inhibits autoimmune disease. Nature Med. 15, 766–773 (2009). This is a good example of how a cell depletion strategy targeting a TNFR molecule can be therapeutic in model systems and pave the path to the development of a clinical reagent.
Cheung, T. C. et al. Polymorphic variants of LIGHT (TNF superfamily-14) alter receptor avidity and bioavailability. J. Immunol. 185, 1949–1958 (2010).
Shaikh, R. B. et al. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J. Immunol. 167, 6330–6337 (2001). This report first identified the pro-inflammatory potential of LIGHT in an in vivo model.
Cohavy, O., Zhou, J., Ware, C. F. & Targan, S. R. LIGHT is constitutively expressed on T & NK cells in the human gut and can be induced by CD2-mediated signaling. J. Immunol. 174, 646–653 (2005).
Doherty, T. A. et al. The tumor necrosis factor family member LIGHT is a target for asthmatic airway remodeling. Nature Med. 17, 596–603 (2011). This work highlighted a new role of LIGHT in chronic lung inflammation.
Croft, M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annu. Rev. Immunol. 28, 57–78 (2010). This article details the history of research on OX40 and the key developments in understanding its importance in immunity.
Salek-Ardakani, S. et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J. Exp. Med. 198, 315–324 (2003).
Seshasayee, D. et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J. Clin. Invest. 117, 3868–3878 (2007).
Ezzat, M. H., Imam, S. S., Shaheen, K. Y. & Elbrhami, E. M. Serum OX40 ligand levels in asthmatic children: a potential biomarker of severity and persistence. Allergy Asthma Proc. 32, 313–318 (2011).
Lei, W. et al. SOX40L: an important inflammatory mediator in adult bronchial asthma. Ann. Acad. Med. Singap. 41, 200–204 (2012).
Oflazoglu, E., Grewal, I. S. & Gerber, H. Targeting CD30/CD30L in oncology and autoimmune and inflammatory diseases. Adv. Exp. Med. Biol. 647, 174–185 (2009).
Withers, D. R. et al. OX40 and CD30 signals in CD4+ T-cell effector and memory function: a distinct role for lymphoid tissue inducer cells in maintaining CD4+ T-cell memory but not effector function. Immunol. Rev. 244, 134–148 (2011).
Winkles, J. A. The TWEAK–Fn14 cytokine–receptor axis: discovery, biology and therapeutic targeting. Nature Rev. Drug Discov. 7, 411–425 (2008).
Burkly, L. C., Michaelson, J. S. & Zheng, T. S. TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses. Immunol. Rev. 244, 99–114 (2011). References 63 and 64 discuss the basic biology of FN14 and how this is translating into clinical targeting.
Bhatnagar, S. & Kumar, A. The TWEAK-Fn14 system: breaking the silence of cytokine-induced skeletal muscle wasting. Curr. Mol. Med. 12, 3–13 (2012).
Kawashima, R. et al. Interleukin-13 damages intestinal mucosa via TWEAK and Fn14 in mice — a pathway associated with ulcerative colitis. Gastroenterology 141, 2119–2129.e8 (2011).
Dohi, T. & Burkly, L. C. The TWEAK/Fn14 pathway as an aggravating and perpetuating factor in inflammatory diseases; focus on inflammatory bowel diseases. J. Leukocyte Biol. 92, 265–279 (2012).
Michaelson, J. S., Wisniacki, N., Burkly, L. C. & Putterman, C. Role of TWEAK in lupus nephritis: a bench-to-bedside review. J. Autoimmun. 39, 130–142 (2012).
Placke, T., Kopp, H. G. & Salih, H. R. Glucocorticoid-induced TNFR-related (GITR) protein and its ligand in antitumor immunity: functional role and therapeutic modulation. Clin. Dev. Immunol. 2010, 239083 (2010).
Nocentini, G., Ronchetti, S., Petrillo, M. G. & Riccardi, C. Pharmacological modulation of GITRL/GITR system: therapeutic perspectives. Br. J. Pharmacol. 165, 2089–2099 (2012). References 69 and 70 highlight the complex biology of GITR and GITRL.
Bae, E. et al. Glucocorticoid-induced tumour necrosis factor receptor-related protein-mediated macrophage stimulation may induce cellular adhesion and cytokine expression in rheumatoid arthritis. Clin. Exp. Immunol. 148, 410–418 (2007).
Bae, E. M. et al. Reverse signaling initiated from GITRL induces NF-κB activation through ERK in the inflammatory activation of macrophages. Mol. Immunol. 45, 523–533 (2008).
Kim, W. J. et al. Comparative analysis of the expression patterns of various TNFSF/TNFRSF in atherosclerotic plaques. Immunol. Invest. 37, 359–373 (2008).
Cuzzocrea, S. et al. Role of glucocorticoid-induced TNF receptor family gene (GITR) in collagen-induced arthritis. FASEB J. 19, 1253–1265 (2005).
Wang, S. et al. Glucocorticoid-induced tumor necrosis factor receptor family-related protein exacerbates collagen-induced arthritis by enhancing the expansion of Th17 cells. Am. J. Pathol. 180, 1059–1067 (2012).
Snell, L. M., Lin, G. H., McPherson, A. J., Moraes, T. J. & Watts, T. H. T-cell intrinsic effects of GITR and 4-1BB during viral infection and cancer immunotherapy. Immunol. Rev. 244, 197–217 (2011). This paper compares and contrasts the activities of 4-1BB and GITR in controlling T cell responsiveness to infectious agents and tumours.
Michel, J., Langstein, J., Hofstadter, F. & Schwarz, H. A soluble form of CD137 (ILA/4-1BB), a member of the TNF receptor family, is released by activated lymphocytes and is detectable in sera of patients with rheumatoid arthritis. Eur. J. Immunol. 28, 290–295 (1998).
Jung, H. W., Choi, S. W., Choi, J. I. & Kwon, B. S. Serum concentrations of soluble 4-1BB and 4-1BB ligand correlated with the disease severity in rheumatoid arthritis. Exp. Mol. Med. 36, 13–22 (2004).
Liu, G. Z. et al. Increased soluble 4-1BB ligand (4-1BBL) levels in peripheral blood of patients with multiple sclerosis. Scand. J. Immunol. 64, 412–419 (2006).
Hamaguchi, Y. et al. Clinical association of serum CD137 (4-1BB) levels in patients with systemic sclerosis. J. Dermatol. Sci. 53, 159–161 (2009).
Maerten, P. et al. Functional expression of 4-1BB (CD137) in the inflammatory tissue in Crohn's disease. Clin. Immunol. 112, 239–246 (2004).
Olofsson, P. S. et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation 117, 1292–1301 (2008).
Vinay, D. S. & Kwon, B. S. TNF superfamily: costimulation and clinical applications. Cell Biol. Int. 33, 453–465 (2009).
Seo, S. K. et al. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nature Med. 10, 1088–1094 (2004). This is an important report showing the potential of agonist targeting of 4-1BB in expanding regulatory cells to cause suppression of autoimmune disease.
Vinay, D. S., Kim, C. H., Choi, B. K. & Kwon, B. S. Origins and functional basis of regulatory CD11c+CD8+ T cells. Eur. J. Immunol. 39, 1552–1563 (2009).
Hong, H. J. et al. A humanized anti-4-1BB monoclonal antibody suppresses antigen-induced humoral immune response in nonhuman primates. J. Immunother. 23, 613–621 (2000).
Suntharalingam, G. et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 355, 1018–1028 (2006).
Shih, D. Q. et al. Insights into TL1A and IBD pathogenesis. Adv. Exp. Med. Biol. 691, 279–288 (2011).
Meylan, F., Richard, A. C. & Siegel, R. M. TL1A and DR3, a TNF family ligand-receptor pair that promotes lymphocyte costimulation, mucosal hyperplasia, and autoimmune inflammation. Immunol. Rev. 244, 188–196 (2011). References 88 and 89 provide an in-depth overview of the biology of TL1A and DR3.
Al-Lamki, R. S. et al. Expression of silencer of death domains and death-receptor-3 in normal human kidney and in rejecting renal transplants. Am. J. Pathol. 163, 401–411 (2003).
Bamias, G. et al. Circulating levels of TNF-like cytokine 1A (TL1A) and its decoy receptor 3 (DcR3) in rheumatoid arthritis. Clin. Immunol. 129, 249–255 (2008).
Bamias, G. et al. Upregulation and nuclear localization of TNF-like cytokine 1A (TL1A) and its receptors DR3 and DcR3 in psoriatic skin lesions. Exp. Dermatol. 20, 725–731 (2011).
Nolte, M. A., van Olffen, R. W., van Gisbergen, K. P. & van Lier, R. A. Timing and tuning of CD27–CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol. Rev. 229, 216–231 (2009). This paper highlights the basic biology of CD27 in controlling T cell activity.
Leibbrandt, A. & Penninger, J. M. Novel functions of RANK(L) signaling in the immune system. Adv. Exp. Med. Biol. 658, 77–94 (2010). This review details new activities of RANK in controlling immune responsiveness.
Leibbrandt, A. & Penninger, J. M. TNF conference 2009: beyond bones — RANKL/RANK in the immune system. Adv. Exp. Med. Biol. 691, 5–22 (2011).
Schmaltz, C. et al. Differential use of Fas ligand and perforin cytotoxic pathways by donor T cells in graft-versus-host disease and graft-versus-leukemia effect. Blood 97, 2886–2895 (2001).
Miwa, K. et al. Therapeutic effect of an anti-Fas ligand mAb on lethal graft-versus-host disease. Int. Immunol. 11, 925–931 (1999).
Ruffin, N. et al. The involvement of epithelial Fas in a human model of graft versus host disease. Transplantation 91, 946–951 (2011).
Tecchio, C. et al. High serum levels of B-lymphocyte stimulator are associated with clinical-pathological features and outcome in classical Hodgkin lymphoma. Br. J. Haematol. 137, 553–559 (2007).
Rennert, P. et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J. Exp. Med. 192, 1677–1684 (2000).
Chiu, A. et al. Hodgkin lymphoma cells express TACI and BCMA receptors and generate survival and proliferation signals in response to BAFF and APRIL. Blood 109, 729–739 (2007).
Guadagnoli, M. et al. Development and characterization of APRIL antagonistic monoclonal antibodies for treatment of B-cell lymphomas. Blood 117, 6856–6865 (2011).
Ansell, S. M. et al. Phase I clinical study of atacicept in patients with relapsed and refractory B-cell non-Hodgkin's lymphoma. Clin. Cancer Res. 14, 1105–1110 (2008).
Rossi, J. F. et al. Atacicept in relapsed/refractory multiple myeloma or active Waldenstrom's macroglobulinemia: a phase I study. Br. J. Cancer 101, 1051–1058 (2009).
Rossi, J. F. Phase I study of atacicept in relapsed/refractory multiple myeloma (MM) and Waldenstrom's macroglobulinemia. Clin. Lymphoma Myeloma Leuk. 11, 136–138 (2011).
Vonderheide, R. H. et al. Clinical activity and immune modulation in cancer patients treated with CP-870893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 25, 876–883 (2007).
Ruter, J., Antonia, S. J., Burris, H. A., Huhn, R. D. & Vonderheide, R. H. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol. Ther. 10, 983–993 (2010).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011). This paper provides new insights into the potential efficacy of CD40 agonists in patients with cancer.
Tai, Y. T. et al. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 64, 2846–2852 (2004).
Kelley, S. K. et al. Preclinical pharmacokinetics, pharmacodynamics, and activity of a humanized anti-CD40 antibody (SGN-40) in rodents and non-human primates. Br. J. Pharmacol. 148, 1116–1123 (2006).
Furman, R. R., Forero-Torres, A., Shustov, A. & Drachman, J. G. A phase I study of dacetuzumab (SGN-40, a humanized anti-CD40 monoclonal antibody) in patients with chronic lymphocytic leukemia. Leuk. Lymphoma 51, 228–235 (2010).
Hussein, M. et al. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica 95, 845–848 (2010).
Tai, Y. T. et al. Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myeloma. Cancer Res. 65, 5898–5906 (2005).
Byrd, J. C. et al. Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia. Leuk. Lymphoma 53, 2136–2142 (2012).
Chowdhury, F., Tutt, A. L., Chan, C., Glennie, M. & Johnson, P. W. Development, validation and application of ELISAs for pharmacokinetic and HACA assessment of a chimeric anti-CD40 monoclonal antibody in human serum. J. Immunol. Methods 363, 1–8 (2010).
Vetto, J. T. et al. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am. J. Surg. 174, 258–265 (1997).
Ramstad, T., Lawnicki, L., Vetto, J. & Weinberg, A. Immunohistochemical analysis of primary breast tumors and tumor-draining lymph nodes by means of the T-cell costimulatory molecule OX-40. Am. J. Surg. 179, 400–406 (2000).
Petty, J. K., He, K., Corless, C. L., Vetto, J. T. & Weinberg, A. D. Survival in human colorectal cancer correlates with expression of the T-cell costimulatory molecule OX-40 (CD134). Am. J. Surg. 183, 512–518 (2002).
Sarff, M. et al. OX40 (CD134) expression in sentinel lymph nodes correlates with prognostic features of primary melanomas. Am. J. Surg. 195, 621–625; discussion 625 (2008).
Weinberg, A. D. et al. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J. Immunother. 29, 575–585 (2006).
Sznol, M. et al. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J. Clin. Oncol. Abstr. 26, 3007 (2008).
Niu, L. et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J. Immunol. 178, 4194–4213 (2007).
Dubrot, J. et al. Treatment with anti-CD137 mAbs causes intense accumulations of liver T cells without selective antitumor immunotherapeutic effects in this organ. Cancer Immunol. Immunother. 59, 1223–1233 (2010).
Wang, J. et al. CD137-mediated pathogenesis from chronic hepatitis to hepatocellular carcinoma in hepatitis B virus-transgenic mice. J. Immunol. 185, 7654–7662 (2010).
Xia, R. et al. TNFSF9 expression in primary biliary cirrhosis and its clinical significance. Cytokine 50, 311–316 (2010).
Fisher, T. S. et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol. Immunother. 61, 1721–1733 (2012).
Schabowsky, R. H. et al. A novel form of 4-1BBL has better immunomodulatory activity than an agonistic anti-4-1BB Ab without Ab-associated severe toxicity. Vaccine 28, 512–522 (2009).
Pastor, F., Kolonias, D., McNamara Ii, J. O. & Gilboa, E. Targeting 4-1BB costimulation to disseminated tumor lesions with bi-specific oligonucleotide aptamers. Mol. Ther. 19, 1878–1886 (2011).
Imai, C. et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 18, 676–684 (2004).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011). This small study shows that human T cells transduced with a chimeric receptor encoding the signalling domain of 4-1BB can be tremendously efficacious in suppressing tumour growth in patients with cancer. This work has rejuvenated the field of cell-based cancer therapy.
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011).
Schaer, D. A., Cohen, A. D. & Wolchok, J. D. Anti-GITR antibodies — potential clinical applications for tumor immunotherapy. Curr. Opin. Invest. Drugs 11, 1378–1386 (2010).
Ponte, J. et al. Characteristics and development of TRX518, an aglycosyl humanized monoclonal antibody (Mab) agonist of huGITR. Clin. Immunol. 135, S96 (2010).
Pruitt, S. K. et al. Enhancement of anti-tumor immunity through local modulation of CTLA-4 and GITR by dendritic cells. Eur. J. Immunol. 41, 3553–3563 (2011).
Law, C. L. et al. Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody–drug conjugates. Cancer Res. 66, 2328–2337 (2006).
McEarchern, J. A. et al. Engineered anti-CD70 antibody with multiple effector functions exhibits in vitro and in vivo antitumor activities. Blood 109, 1185–1192 (2007).
McEarchern, J. A. et al. Preclinical characterization of SGN-70, a humanized antibody directed against CD70. Clin. Cancer Res. 14, 7763–7772 (2008).
Ryan, M. C. et al. Targeting pancreatic and ovarian carcinomas using the auristatin-based anti-CD70 antibody–drug conjugate SGN-75. Br. J. Cancer 103, 676–684 (2010).
Vitale, L. et al. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin. Cancer Res. 18, 3812–3821 (2012).
Van Nuffel, A. M. et al. Intravenous and intradermal TriMix-dendritic cell therapy results in a broad T-cell response and durable tumor response in a chemorefractory stage IV-M1c melanoma patient. Cancer Immunol. Immunother. 61, 1033–1043 (2011).
Falini, B. et al. Response of refractory Hodgkin's disease to monoclonal anti-CD30 immunotoxin. Lancet 339, 1195–1196 (1992).
Terenzi, A. et al. Anti-CD30 (BER=H2) immunotoxins containing the type-1 ribosome-inactivating proteins momordin and PAP-S (pokeweed antiviral protein from seeds) display powerful antitumour activity against CD30+ tumour cells in vitro and in SCID mice. Br. J. Haematol. 92, 872–879 (1996).
Wahl, A. F. et al. The anti-CD30 monoclonal antibody SGN-30 promotes growth arrest and DNA fragmentation in vitro and affects antitumor activity in models of Hodgkin's disease. Cancer Res. 62, 3736–3742 (2002).
Forero-Torres, A. et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br. J. Haematol. 146, 171–179 (2009).
Duvic, M. et al. A phase II study of SGN-30 in cutaneous anaplastic large cell lymphoma and related lymphoproliferative disorders. Clin. Cancer Res. 15, 6217–6224 (2009).
Bartlett, N. L. et al. A phase 1 multidose study of SGN-30 immunotherapy in patients with refractory or recurrent CD30+ hematologic malignancies. Blood 111, 1848–1854 (2008).
Francisco, J. A. et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102, 1458–1465 (2003).
Younes, A. et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J. Clin. Oncol. 30, 2183–2189 (2012).
Fanale, M. A. et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin. Cancer Res. 18, 248–255 (2012). The studies reported in references 149 and 150 (and in prior references on the clinical development of CD30-targeting antibodies) led to the clinical approval of brentuximab vedotin for the treatment of Hodgkin's lymphoma and ALCL.
Blum, K. A. et al. Serious pulmonary toxicity in patients with Hodgkin's lymphoma with SGN-30, gemcitabine, vinorelbine, and liposomal doxorubicin is associated with an FcγRIIIa-158 V/F polymorphism. Ann. Oncol. 21, 2246–2254 (2010).
Savoldo, B. et al. Epstein Barr virus-specific cytotoxic T lymphocytes expressing the anti-CD30ζ artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood 110, 2620–2630 (2007).
Di Stasi, A. et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 113, 6392–6402 (2009).
Huang, Y. & Sheikh, M. S. TRAIL death receptors and cancer therapeutics. Toxicol. Appl. Pharmacol. 224, 284–289 (2007).
Fox, N. L., Humphreys, R., Luster, T. A., Klein, J. & Gallant, G. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor-1 and receptor-2 agonists for cancer therapy. Expert Opin. Biol. Ther. 10, 1–18 (2010). This is a comprehensive review of clinical trial results and differences in specific drugs targeting the TRAIL cytokine system in patients with cancer.
Gerspach, J., Pfizenmaier, K. & Wajant, H. Therapeutic targeting of CD95 and the TRAIL death receptors. Recent Pat. Anticancer Drug Discov. 6, 294–310 (2011).
Ashkenazi, A., Holland, P. & Eckhardt, S. G. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL). J. Clin. Oncol. 26, 3621–3630 (2008).
Hellwig, C. T. & Rehm, M. TRAIL signaling and synergy mechanisms used in TRAIL-based combination therapies. Mol. Cancer Ther. 11, 3–13 (2012).
Soria, J. C. et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J. Clin. Oncol. 29, 4442–4451 (2011).
Shepard, B. D. & Badley, A. D. The biology of TRAIL and the role of TRAIL-based therapeutics in infectious diseases. Antiinfect. Agents Med. Chem. 8, 87–101 (2009).
Younes, A. et al. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin's lymphoma. Br. J. Cancer 103, 1783–1787 (2010).
Trarbach, T. et al. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br. J. Cancer 102, 506–512 (2010).
Bajaj, M. & Heath, E. I. Conatumumab: a novel monoclonal antibody against death receptor 5 for the treatment of advanced malignancies in adults. Expert Opin. Biol. Ther. 11, 1519–1524 (2011).
Demetri, G. D. et al. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: a phase I/II open-label and double-blind study. Eur. J. Cancer 48, 547–563 (2012).
Kindler, H. L. et al. A randomized, placebo-controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Ann. Oncol. 23, 2834–2842 (2012).
Camidge, D. R. et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin. Cancer Res. 16, 1256–1263 (2010).
Wilson, N. S. et al. An Fcγ receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer Cell 19, 101–113 (2011). This report shows that, in mice, the antitumour activity of a TRAILR2-specific monoclonal antibody currently in clinical trials (drozitumab) is augmented through its interaction with leukocytes expressing both activating and inhibitory FcγRs.
Buchsbaum, D. J. et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin. Cancer Res. 9, 3731–3741 (2003).
Saleh, M. N. et al. A phase I study of CD-1008 (humanized monoclonal antibody targeting death receptor 5 or DR5), administered weekly to patients with advanced solid tuors or lymphomas. J. Clin. Oncol. Abstr. 26, 3537 (2008).
Sharma, S. et al. Phase I trial of LBY135, a monoclonal antibody agonist to DR5, alone and in combination with capecitabine in advanced solid tumors. J. Clin. Oncol. Abstr. 26, 3538 (2008).
Culp, P. A. et al. Antibodies to TWEAK receptor inhibit human tumor growth through dual mechanisms. Clin. Cancer Res. 16, 497–508 (2010).
Michaelson, J. S. et al. Development of an Fn14 agonistic antibody as an anti-tumor agent. MAbs 3, 362–375 (2011).
Michaelson, J. S. et al. The anti-Fn14 antibody BIIB036 inhibits tumor growth in xenografts and patient derived primary tumor models and enhances efficacy of chemotherapeutic agents in multiple xenograft models. Cancer Biol. Ther. 13, 812–821 (2012).
Chao, D. T. et al. Expression of TweakR in breast cancer and preclinical activity of enavatuzumab, a humanized anti-TweakR mAb. J. Cancer Res. Clin. Oncol. 17 Oct 2012 (doi:10.1007/s00432-012-1332-x).
Yu, P. & Fu, Y. X. Targeting tumors with LIGHT to generate metastasis-clearing immunity. Cytokine Growth Factor Rev. 19, 285–294 (2008).
Zhu, M. & Fu, Y. X. The role of core TNF/LIGHT family members in lymph node homeostasis and remodeling. Immunol. Rev. 244, 75–84 (2011). Together with reference 6, references 175 and 176 provide an overview of the latest developments in research on HVEM, LIGHT and their binding partners that relate to inflammatory disease and cancer.
Tamada, K. et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nature Med. 6, 283–289 (2000).
Lukashev, M. et al. Targeting the lymphotoxin-β receptor with agonist antibodies as a potential cancer therapy. Cancer Res. 66, 9617–9624 (2006).
Haybaeck, J. et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 16, 295–308 (2009).
Ammirante, M., Luo, J. L., Grivennikov, S., Nedospasov, S. & Karin, M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464, 302–305 (2010).
Bouillet, P. & O'Reilly, L. A. CD95, BIM and T cell homeostasis. Nature Rev. Immunol. 9, 514–519 (2009).
Strasser, A., Jost, P. J. & Nagata, S. The many roles of FAS receptor signaling in the immune system. Immunity 30, 180–192 (2009). References 181 and 182 describe the activities of FAS in controlling immune responses.
Lin, W. W. & Hsieh, S. L. Decoy receptor 3: a pleiotropic immunomodulator and biomarker for inflammatory diseases, autoimmune diseases and cancer. Biochem. Pharmacol. 81, 838–847 (2011).
Schungel, S. et al. The strength of the Fas ligand signal determines whether hepatocytes act as type 1 or type 2 cells in murine livers. Hepatology 50, 1558–1566 (2009).
Verbrugge, I. et al. Combining radiotherapy with APO010 in cancer treatment. Clin. Cancer Res. 15, 2031–2038 (2009).
Eisele, G. et al. APO010, a synthetic hexameric CD95 ligand, induces human glioma cell death in vitro and in vivo. Neuro-oncology 13, 155–164 (2011).
Lacey, D. L. et al. Bench to bedside: elucidation of the OPG–RANK–RANKL pathway and the development of denosumab. Nature Rev. Drug Discov. 11, 401–419 (2012). This is a comprehensive review of the research and clinical history resulting in the development of denosumab for the treatment of bone-related disorders.
Johnson-Pais, T. L. et al. Identification of a novel tandem duplication in exon 1 of the TNFRSF11A gene in two unrelated patients with familial expansile osteolysis. J. Bone Miner. Res. 18, 376–380 (2003).
Whyte, M. P. & Hughes, A. E. Expansile skeletal hyperphosphatasia is caused by a 15-base pair tandem duplication in TNFRSF11A encoding RANK and is allelic to familial expansile osteolysis. J. Bone Miner. Res. 17, 26–29 (2002).
Nakatsuka, K., Nishizawa, Y. & Ralston, S. H. Phenotypic characterization of early onset Paget's disease of bone caused by a 27-bp duplication in the TNFRSF11A gene. J. Bone Miner. Res. 18, 1381–1385 (2003).
Whyte, M. P. et al. Osteoprotegerin deficiency and juvenile Paget's disease. N. Engl. J. Med. 347, 175–184 (2002).
Cummings, S. R. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361, 756–765 (2009).
Papapoulos, S. et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J. Bone Miner. Res. 27, 694–701 (2012).
McClung, M. R. et al. Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos. Int. 10 Jul 2012 (doi:10.1007/s00198-012-2052-4).
Sharp, J. T. et al. Denosumab prevents metacarpal shaft cortical bone loss in patients with erosive rheumatoid arthritis. Arthritis Care Res. 62, 537–544 (2010).
Deodhar, A. et al. Denosumab-mediated increase in hand bone mineral density associated with decreased progression of bone erosion in rheumatoid arthritis patients. Arthritis Care Res. 62, 569–574 (2010).
Bekker, P. J. et al. The effect of a single dose of osteoprotegerin in postmenopausal women. J. Bone Miner. Res. 16, 348–360 (2001).
Dougall, W. C. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin. Cancer Res. 18, 326–335 (2012).
Body, J. J. et al. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer 97, 887–892 (2003).
Hill, R. Blocking the effects of NGF as a route to safe and effective pain relief — fact or fancy? Pain 152, 2200–2201 (2011).
Holmes, D. Anti-NGF painkillers back on track? Nature Rev. Drug Discov. 11, 337–338 (2012). References 200 and 201 discuss the potential of targeting NGF for pain.
Lane, N. E. et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 363, 1521–1531 (2010).
Katz, N. et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 152, 2248–2258 (2011).
Nagashima, H., Suzuki, M., Araki, S., Yamabe, T. & Muto, C. Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis Cartilage 19, 1405–1412 (2011).
Schnitzer, T. J. et al. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthritis Cartilage 19, 639–646 (2011).
Brown, M. T. et al. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J. Pain 13, 790–798 (2012).
Tracey, D., Klareskog, L., Sasso, E. H., Salfeld, J. G. & Tak, P. P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol. Ther. 117, 244–279 (2008).
Lichtenstein, G. R., Yan, S., Bala, M., Blank, M. & Sands, B. E. Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn's disease. Gastroenterology 128, 862–869 (2005).
Sandborn, W. J. et al. Etanercept for active Crohn's disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology 121, 1088–1094 (2001).
Ware, C. F. & Sedy, J. R. TNF superfamily networks: bidirectional and interference pathways of the herpesvirus entry mediator (TNFSF14). Curr. Opin. Immunol. 23, 627–631 (2011).
Gregory, A. P. et al. TNF receptor 1 genetic risk mirrors outcome of anti-TNF therapy in multiple sclerosis. Nature 488, 508–511 (2012).
van Oosten, B. W. et al. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 47, 1531–1534 (1996).
The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology 53, 457–465 (1999).
Salek-Ardakani, S. & Croft, M. Tumor necrosis factor receptor/tumor necrosis factor family members in antiviral CD8 T-cell immunity. J. Interferon Cytokine Res. 30, 205–218 (2010).
Furst, D. E. The risk of infections with biologic therapies for rheumatoid arthritis. Semin. Arthritis Rheum. 39, 327–346 (2010).
Kim, S. Y. & Solomon, D. H. Tumor necrosis factor blockade and the risk of viral infection. Nature Rev. Rheumatol. 6, 165–174 (2010).
Wang, X. Y., Zuo, D., Sarkar, D. & Fisher, P. B. Blockade of cytotoxic T-lymphocyte antigen-4 as a new therapeutic approach for advanced melanoma. Expert Opin. Pharmacother. 12, 2695–2706 (2011).
Weinblatt, M. et al. Selective costimulation modulation using abatacept in patients with active rheumatoid arthritis while receiving etanercept: a randomised clinical trial. Ann. Rheum. Dis. 66, 228–234 (2007).
Acknowledgements
M.C. is supported by the following grants from the US National Institutes of Health (NIH): CA91837, AI49453, AI089624, AI100905 and AI070535. C.F.W. is supported by NIH grants AI33068, AI48073 and CA164679; and C.A.B. is supported by an NIH grant (AI101403) and an American Heart Association (AHA) grant (7510081). This is publication #1426 from the La Jolla Institute for Allergy and Immunology.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
M.C. and C.F.W. have licensed patents on several TNFSF and TNFRSF molecules. C.A.B. declares no competing financial interests.
Supplementary information
Related links
Glossary
- ACR-50 score
-
American College of Rheumatology score indicating a 50% improvement in symptoms.
Rights and permissions
About this article
Cite this article
Croft, M., Benedict, C. & Ware, C. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov 12, 147–168 (2013). https://doi.org/10.1038/nrd3930
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3930
This article is cited by
-
TBK1, a prioritized drug repurposing target for amyotrophic lateral sclerosis: evidence from druggable genome Mendelian randomization and pharmacological verification in vitro
BMC Medicine (2024)
-
Autoinhibitory structure of preligand association state implicates a new strategy to attain effective DR5 receptor activation
Cell Research (2023)
-
Development of a 1:1-binding biparatopic anti-TNFR2 antagonist by reducing signaling activity through epitope selection
Communications Biology (2023)
-
A novel disulfidptosis-related immune checkpoint genes signature: forecasting the prognosis of hepatocellular carcinoma
Journal of Cancer Research and Clinical Oncology (2023)
-
Comprehensive analysis of a TNF family based-signature in diffuse gliomas with regard to prognosis and immune significance
Cell Communication and Signaling (2022)