Key Points
-
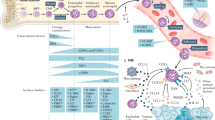
The activation of eosinophils leads to the release of preformed and newly synthesized products including cytokines, chemokines, lipid mediators and cytotoxic granule proteins that can initiate, escalate and sustain local inflammatory and remodelling responses.
-
Eosinophil secondary granules are primarily composed of highly charged basic proteins, including eosinophil granule major basic proteins, eosinophil cationic protein, eosinophil-derived neurotoxin and eosinophil peroxidase. In addition, eosinophil granules contain a plethora of preformed cytokines, chemokines, enzymes and growth factors, resulting in the diverse biological activity of eosinophils in infection and inflammation.
-
Although glucocorticoids are very effective at reducing eosinophil numbers in the blood and tissue, patients often experience harmful side effects and develop resistance. Therapy directed against the eosinophil growth factor interleukin-5 (IL-5) has been tested in clinical trials for efficacy in several eosinophil-associated disorders and looks promising. However, the reduction in tissue eosinophilia and improvement in symptoms has been variable and dependent on patient phenotypes (subgroups). Thus, additional approaches for selecting patients and new drugs based on an improved understanding of the mechanism of eosinophilia and the effector functions of eosinophils are needed.
-
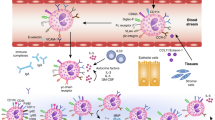
Preclinical studies suggest that inhibition of eosinophil migration from the bloodstream into tissues has therapeutic potential, but there has been limited clinical success to date with current targets and approaches. The lack of effectiveness may be due to the complex regulation of eosinophil recruitment into inflammatory tissues.
-
Preclinical studies that are focused on promoting eosinophil apoptosis support continued investigations into sialic acid-binding immunoglobulin-like lectin 8 (SIGLEC-8)-targeted therapies, as engagement of SIGLEC-8 results in selective apoptosis of eosinophils.
-
The treatment of patients with allergic asthma using omalizumab, a recombinant monoclonal antibody that binds to immunoglobulin E, results in a reduction in blood and tissue eosinophilia through an as yet unknown mechanism. Studies that are focused on the mechanism of this reduction are needed and may yield new therapeutic targets.
-
Post-hoc analyses of clinical trial results have emphasized the importance of subphenotyping patients to identify those patients who are likely to receive the most therapeutic benefit from a specific agent. There is a need to identify subgroups of patients with eosinophilic disorders to predict treatment responses on the basis of a patient's genetics or gene expression profiles of affected organs in order to tailor the therapy for their eosinophil-associated disease.
Abstract
Eosinophils can regulate local immune and inflammatory responses, and their accumulation in the blood and tissue is associated with several inflammatory and infectious diseases. Thus, therapies that target eosinophils may help control diverse diseases, including atopic disorders such as asthma and allergy, as well as diseases that are not primarily associated with eosinophils, such as autoimmunity and malignancy. Eosinophil-targeted therapeutic agents that are aimed at blocking specific steps involved in eosinophil development, migration and activation have recently entered clinical testing and have produced encouraging results and insights into the role of eosinophils. In this Review, we describe recent advances in the development of first-generation eosinophil-targeted therapies and highlight strategies for using personalized medicine to treat eosinophilic disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lee, J. J. et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305, 1773–1776 (2004).
Humbles, A. A. et al. A critical role for eosinophils in allergic airways remodeling. Science 305, 1776–1779 (2004).
Castro, M. et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am. J. Respir. Crit. Care Med. 184, 1125–1132 (2011).
Haldar, P. et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 360, 973–984 (2009). This study reveals an important role for eosinophils in asthma exacerbations in patients with refractory eosinophilic asthma. Treatment with mepolizumab resulted in significantly fewer severe exacerbations in a population of patients with a history of recurrent severe exacerbations.
Jacobsen, E. A., Helmers, R. A., Lee, J. J. & Lee, N. A. The expanding role(s) of eosinophils in health and disease. Blood 120, 3882–3890 (2012). This review highlights studies that have implicated a role for eosinophils in several diseases and healthhomeostasis.
Lee, J. J., Jacobsen, E. A., McGarry, M. P., Schleimer, R. P. & Lee, N. A. Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy 40, 563–575 (2010).
Lowe, D., Jorizzo, J. & Hutt, M. S. Tumour-associated eosinophilia: a review. J. Clin. Pathol. 34, 1343–1348 (1981).
Cormier, S. A. et al. Pivotal advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J. Leukoc. Biol. 79, 1131–1139 (2006).
Simson, L. et al. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J. Immunol. 178, 4222–4229 (2007).
Chu, V. T. et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nature Immunol. 12, 151–159 (2011). This study documents a novel role for eosinophils in promoting the survival of long-lived plasma cells in the bone marrow.
Chu, V. T. & Berek, C. Immunization induces activation of bone marrow eosinophils required for plasma cell survival. Eur. J. Immunol. 42, 130–137 (2012).
Hruz, P. et al. Escalating incidence of eosinophilic esophagitis: a 20-year prospective, population-based study in Olten County, Switzerland. J. Allergy Clin. Immunol. 128, 1349–1350 (2011).
Bohm, M. et al. Mucosal eosinophilia: prevalence and racial/ethnic differences in symptoms and endoscopic findings in adults over 10 years in an urban hospital. J. Clin. Gastroenterol. 46, 567–574 (2011).
Mori, Y. et al. Identification of the human eosinophil lineage-committed progenitor: revision of phenotypic definition of the human common myeloid progenitor. J. Exp. Med. 206, 183–193 (2009). This study identified the lineage-committed eosinophil progenitor in human bone marrow and also showed increased numbers of eosinophil progenitors in patients with eosinophilia.
Iwasaki, H. et al. Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J. Exp. Med. 201, 1891–1897 (2005).
Sehmi, R. et al. Allergen-induced increases in IL-5 receptor α-subunit expression on bone marrow-derived CD34+ cells from asthmatic subjects. A novel marker of progenitor cell commitment towards eosinophilic differentiation. J. Clin. Invest. 100, 2466–2475 (1997).
Hogan, S. P. et al. Eosinophils: biological properties and role in health and disease. Clin. Exp. Allergy 38, 709–750 (2008).
Ahlstrom-Emanuelsson, C. A., Greiff, L., Andersson, M., Persson, C. G. & Erjefalt, J. S. Eosinophil degranulation status in allergic rhinitis: observations before and during seasonal allergen exposure. Eur. Respir. J. 24, 750–757 (2004).
Filley, W. V., Holley, K. E., Kephart, G. M. & Gleich, G. J. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet 2, 11–16 (1982).
Kephart, G. M. et al. Marked deposition of eosinophil-derived neurotoxin in adult patients with eosinophilic esophagitis. Am. J. Gastroenterol. 105, 298–307 (2010).
Noguchi, H., Kephart, G. M., Colby, T. V. & Gleich, G. J. Tissue eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am. J. Pathol. 140, 521–528 (1992).
Chung, H. L. et al. Deposition of eosinophil-granule major basic protein and expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in the mucosa of the small intestine in infants with cow's milk-sensitive enteropathy. J. Allergy Clin. Immunol. 103, 1195–1201 (1999).
Brottman, G. M., Regelmann, W. E., Slungaard, A. & Wangensteen, O. D. Effect of eosinophil peroxidase on airway epithelial permeability in the guinea pig. Pediatr. Pulmonol. 21, 159–166 (1996).
Gleich, G. J., Frigas, E., Loegering, D. A., Wassom, D. L. & Steinmuller, D. Cytotoxic properties of the eosinophil major basic protein. J. Immunol. 123, 2925–2927 (1979).
Tai, P. C., Hayes, D. J., Clark, J. B. & Spry, C. J. Toxic effects of human eosinophil products on isolated rat heart cells in vitro. Biochem. J. 204, 75–80 (1982).
Rosenberg, H. F. Eosinophil-derived neurotoxin/RNase 2: connecting the past, the present and the future. Curr. Pharm. Biotechnol. 9, 135–140 (2008).
Domachowske, J. B., Dyer, K. D., Bonville, C. A. & Rosenberg, H. F. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J. Infect. Dis. 177, 1458–1464 (1998).
Hernnas, J. et al. Eosinophil cationic protein alters proteoglycan metabolism in human lung fibroblast cultures. Eur. J. Cell Biol. 59, 352–363 (1992).
Muniz, V. S., Weller, P. F. & Neves, J. S. Eosinophil crystalloid granules: structure, function, and beyond. J. Leukoc. Biol. 92, 281–288 (2012).
Meagher, L. C., Cousin, J. M., Seckl, J. R. & Haslett, C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J. Immunol. 156, 4422–4428 (1996).
Druilhe, A., Letuve, S. & Pretolani, M. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis 8, 481–495 (2003).
Her, E., Frazer, J., Austen, K. F. & Owen, W. F. Jr. Eosinophil hematopoietins antagonize the programmed cell death of eosinophils. Cytokine and glucocorticoid effects on eosinophils maintained by endothelial cell-conditioned medium. J. Clin. Invest. 88, 1982–1987 (1991).
Jain, N. et al. Imatinib has limited therapeutic activity for hypereosinophilic syndrome patients with unknown or negative PDGFRα mutation status. Leuk. Res. 33, 837–839 (2009).
Ogbogu, P. U. et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J. Allergy Clin. Immunol. 124, 1319–1325 (2009).
Hamelmann, E. et al. Anti-interleukin 5 but not anti-IgE prevents airway inflammation and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 160, 934–941 (1999).
Rothenberg, M. E. et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N. Engl. J. Med. 358, 1215–1228 (2008). This clinical trial showed that administration of mepolizumab to steroid-dependent patients with hypereosinophilic syndrome resulted in significant reductions in the dose of corticosteroid needed and also resulted in corticosteroid discontinuation.
Stein, M. L. et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J. Allergy Clin. Immunol. 118, 1312–1319 (2006).
Pavord, I. D. et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 380, 651–659 (2012).
Gong, L. & Wilhelm, R. S. CCR3 antagonists: a survey of the patent literature. Expert Opin. Ther. Pat. 19, 1109–1132 (2009).
Pease, J. E. & Williams, T. J. Eotaxin and asthma. Curr. Opin. Pharmacol. 1, 248–253 (2001).
Tenscher, K., Metzner, B., Schopf, E., Norgauer, J. & Czech, W. Recombinant human eotaxin induces oxygen radical production, Ca2+-mobilization, actin reorganization, and CD11b upregulation in human eosinophils via a pertussis toxin-sensitive heterotrimeric guanine nucleotide-binding protein. Blood 88, 3195–3199 (1996).
Kampen, G. T. et al. Eotaxin induces degranulation and chemotaxis of eosinophils through the activation of ERK2 and p38 mitogen-activated protein kinases. Blood 95, 1911–1917 (2000).
Fulkerson, P. C., Fischetti, C. A. & Rothenberg, M. E. Eosinophils and CCR3 regulate interleukin-13 transgene-induced pulmonary remodeling. Am. J. Pathol. 169, 2117–2126 (2006).
Fulkerson, P. C. et al. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc. Natl Acad. Sci. USA 103, 16418–16423 (2006).
Ahrens, R. et al. Intestinal macrophage/epithelial cell-derived CCL11/eotaxin-1 mediates eosinophil recruitment and function in pediatric ulcerative colitis. J. Immunol. 181, 7390–7399 (2008).
Waddell, A. et al. Colonic eosinophilic inflammation in experimental colitis is mediated by Ly6Chigh CCR2+ inflammatory monocyte/macrophage-derived CCL11. J. Immunol. 186, 5993–6003 (2011).
Ying, S. et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur. J. Immunol. 27, 3507–3516 (1997).
Wegmann, M. et al. Effects of a low-molecular-weight CCR-3 antagonist on chronic experimental asthma. Am. J. Respir. Cell. Mol. Biol. 36, 61–67 (2007).
Komai, M. et al. A novel CC-chemokine receptor 3 antagonist, Ki19003, inhibits airway eosinophilia and subepithelial/peribronchial fibrosis induced by repeated antigen challenge in mice. J. Pharmacol. Sci. 112, 203–213 (2010).
Ding, C., Li, J. & Zhang, X. Bertilimumab Cambridge Antibody Technology Group. Curr. Opin. Investig. Drugs 5, 1213–1218 (2004).
Blanchard, C. et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J. Clin. Invest. 116, 536–547 (2006). This study was the first to demonstrate a distinct transcript signature in oesophageal biopsy specimens from patients with eosinophilic oesophagitis. The eosinophil-specific chemoattractant CCL26 gene was the most highly induced gene,suggesting that CCL26 has a crucial role in disease pathogenesis.
Zuo, L. et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13Rα2-inhibited pathway. J. Immunol. 185, 660–669 (2010).
Mattes, J. et al. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J. Exp. Med. 195, 1433–1444 (2002).
Nakajima, H., Sano, H., Nishimura, T., Yoshida, S. & Iwamoto, I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J. Exp. Med. 179, 1145–1154 (1994).
Henderson, W. R. Jr et al. Blockade of CD49d (α4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J. Clin. Invest. 100, 3083–3092 (1997).
Okigami, H. et al. Inhibition of eosinophilia in vivo by a small molecule inhibitor of very late antigen (VLA)-4. Eur. J. Pharmacol. 559, 202–209 (2007).
Abbas, M. et al. Hypereosinophilia in patients with multiple sclerosis treated with natalizumab. Neurology 77, 1561–1564 (2011).
Schleimer, R. P. et al. Role of human basophils and mast cells in the pathogenesis of allergic diseases. J. Allergy Clin. Immunol. 76, 369–374 (1985).
Schratl, P. et al. The role of the prostaglandin D2 receptor, DP, in eosinophil trafficking. J. Immunol. 179, 4792–4799 (2007).
Royer, J. F. et al. A novel antagonist of CRTH2 blocks eosinophil release from bone marrow, chemotaxis and respiratory burst. Allergy 62, 1401–1409 (2007).
Hirai, H. et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 193, 255–261 (2001).
Kagawa, S. et al. Role of prostaglandin D2 receptor CRTH2 in sustained eosinophil accumulation in the airways of mice with chronic asthma. Int. Arch. Allergy Immunol. 155 (Suppl. 1), 6–11 (2011).
Heinemann, A., Schuligoi, R., Sabroe, I., Hartnell, A. & Peskar, B. A. δ12-prostaglandin J2, a plasma metabolite of prostaglandin D2, causes eosinophil mobilization from the bone marrow and primes eosinophils for chemotaxis. J. Immunol. 170, 4752–4758 (2003).
Sugimoto, H. et al. An orally bioavailable small molecule antagonist of CRTH2, ramatroban (BAY u3405), inhibits prostaglandin D2-induced eosinophil migration in vitro. J. Pharmacol. Exp. Ther. 305, 347–352 (2003).
Schuligoi, R. et al. CRTH2 and D-type prostanoid receptor antagonists as novel therapeutic agents for inflammatory diseases. Pharmacology 85, 372–382 (2010).
Barnes, N. et al. A randomized, double-blind, placebo-controlled study of the CRTH2 antagonist OC000459 in moderate persistent asthma. Clin. Exp. Allergy 42, 38–48 (2012).
Green, R. H. et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 360, 1715–1721 (2002).
Straumann, A. et al. 856 treatment of eosinophilic esophagitis with the CRTH2-antagonist Oc000459: a novel therapeutic principle. Gastroenterology 142 (Suppl. 1), 147 (2012).
O'Reilly, M. et al. Identification of a histamine H4 receptor on human eosinophils — role in eosinophil chemotaxis. J. Recept. Signal Transduct. Res. 22, 431–448 (2002).
Ling, P. et al. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br. J. Pharmacol. 142, 161–171 (2004).
Shin, N. et al. INCB38579, a novel and potent histamine H4 receptor small molecule antagonist with anti-inflammatory pain and anti-pruritic functions. Eur. J. Pharmacol. 675, 47–56 (2012).
Zampeli, E. & Tiligada, E. The role of histamine H4 receptor in immune and inflammatory disorders. Br. J. Pharmacol. 157, 24–33 (2009).
Yu, S., Stahl, E., Li, Q. & Ouyang, A. Antigen inhalation induces mast cells and eosinophils infiltration in the guinea pig esophageal epithelium involving histamine-mediated pathway. Life Sci. 82, 324–330 (2008).
Thurmond, R. L., Gelfand, E. W. & Dunford, P. J. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nature Rev. Drug Discov. 7, 41–53 (2008).
Finkelman, F. D., Hogan, S. P., Hershey, G. K., Rothenberg, M. E. & Wills-Karp, M. Importance of cytokines in murine allergic airway disease and human asthma. J. Immunol. 184, 1663–1674 (2010).
Sherrill, J. D. & Rothenberg, M. E. Genetic dissection of eosinophilic esophagitis provides insight into disease pathogenesis and treatment strategies. J. Allergy Clin. Immunol. 128, 23–32 (2011).
Pope, S. M. et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J. Allergy Clin. Immunol. 108, 594–601 (2001).
Pope, S. M. et al. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J. Biol. Chem. 280, 13952–13961 (2005).
Blanchard, C. et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J. Allergy Clin. Immunol. 120, 1292–1300 (2007).
Huang, S. K. et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J. Immunol. 155, 2688–2694 (1995).
Prieto, J. et al. Increased interleukin-13 mRNA expression in bronchoalveolar lavage cells of atopic patients with mild asthma after repeated low-dose allergen provocations. Respir. Med. 94, 806–814 (2000).
Gauvreau, G. M. et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am. J. Respir. Crit. Care Med. 183, 1007–1014 (2011).
Corren, J. et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 365, 1088–1098 (2011). This study, involving patients with poorly controlled asthma, demonstrated that treatment with lebrikizumab improved lung function, especially in patients with higher peripheral blood eosinophil counts and periostin levels.
Nicholson, G. C. et al. The effects of an anti-IL-13 mAb on cytokine levels and nasal symptoms following nasal allergen challenge. J. Allergy Clin. Immunol. 128, 800–807 (2011).
Tomkinson, A. et al. A murine IL-4 receptor antagonist that inhibits IL-4- and IL-13-induced responses prevents antigen-induced airway eosinophilia and airway hyperresponsiveness. J. Immunol. 166, 5792–5800 (2001).
Munitz, A., Brandt, E. B., Mingler, M., Finkelman, F. D. & Rothenberg, M. E. Distinct roles for IL-13 and IL-4 via IL-13 receptor α1 and the type II IL-4 receptor in asthma pathogenesis. Proc. Natl Acad. Sci. USA 105, 7240–7245 (2008).
Corren, J. et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Rα antagonist, in patients with asthma. Am. J. Respir. Crit. Care Med. 181, 788–796 (2010).
Wenzel, S., Wilbraham, D., Fuller, R., Getz, E. B. & Longphre, M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet 370, 1422–1431 (2007).
Nair, P. et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N. Engl. J. Med. 360, 985–993 (2009).
Catley, M. C., Coote, J., Bari, M. & Tomlinson, K. L. Monoclonal antibodies for the treatment of asthma. Pharmacol. Ther. 132, 333–351 (2011).
Rothenberg, M. E. & Hogan, S. P. The eosinophil. Annu. Rev. Immunol. 24, 147–174 (2006).
Takatsu, K. Interleukin-5 and IL-5 receptor in health and diseases. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 87, 463–485 (2011).
Molfino, N. A., Gossage, D., Kolbeck, R., Parker, J. M. & Geba, G. P. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin. Exp. Allergy 42, 712–737 (2012).
Neves, J. S. & Weller, P. F. Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology. Curr. Opin. Immunol. 21, 694–699 (2009). This review highlights extracellular eosinophil granules that function as independent organelles after eosinophil lysis.
Stern, M., Meagher, L., Savill, J. & Haslett, C. Apoptosis in human eosinophils. Programmed cell death in the eosinophil leads to phagocytosis by macrophages and is modulated by IL-5. J. Immunol. 148, 3543–3549 (1992).
Tai, P. C., Sun, L. & Spry, C. J. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin. Exp. Immunol. 85, 312–316 (1991).
Yamaguchi, Y. et al. Analysis of the survival of mature human eosinophils: interleukin-5 prevents apoptosis in mature human eosinophils. Blood 78, 2542–2547 (1991).
Assa'ad, A. H. et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 141, 1593–1604 (2011).
Straumann, A. et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut 59, 21–30 (2010).
Gevaert, P. et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J. Allergy Clin. Immunol. 128, 989–995 (2011).
Spergel, J. M. et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 129, 456–463.e3 (2012).
Kips, J. C. et al. Effect of SCH55700, a humanized anti-human interleukin-5 antibody, in severe persistent asthma: a pilot study. Am. J. Respir. Crit. Care Med. 167, 1655–1659 (2003).
Kolbeck, R. et al. MEDI-563, a humanized anti-IL-5 receptor α mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 125, 1344–1353 (2010).
Busse, W. W. et al. Safety profile, pharmacokinetics, and biologic activity of MEDI-563, an anti-IL-5 receptor α antibody, in a phase I study of subjects with mild asthma. J. Allergy Clin. Immunol. 125, 1237–1244 (2010). This paper reports the results ofan initial Phase I clinical trial that demonstrated an acceptable safety profile and marked reduction in peripheral blood eosinophil counts after treatment with an antibody targeting IL-5Rα.
Imaoka, H. et al. TPI ASM8 reduces eosinophil progenitors in sputum after allergen challenge. Clin. Exp. Allergy 41, 1740–1746 (2011).
Kole, R., Krainer, A. R. & Altman, S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nature Rev. Drug Discov. 11, 125–140 (2012).
Burnett, J. C. & Rossi, J. J. RNA-based therapeutics: current progress and future prospects. Chem. Biol. 19, 60–71 (2012).
Graziewicz, M. A. et al. An endogenous TNF-α antagonist induced by splice-switching oligonucleotides reduces inflammation in hepatitis and arthritis mouse models. Mol. Ther. 16, 1316–1322 (2008).
Bochner, B. S. Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin. Exp. Allergy 39, 317–324 (2009).
Nutku, E., Aizawa, H., Hudson, S. A. & Bochner, B. S. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood 101, 5014–5020 (2003). This study demonstrates the induction of eosinophil apoptosis following the crosslinking of SIGLEC-8 with antibodies and shows that cytokines that promote eosinophil survival (such as IL-5 and GM-CSF) enhance the sensitivity of eosinophils to SIGLEC-8-induced apoptosis.This suggests that targeting SIGLEC-8 may be useful in reducing eosinophil numbers.
Zhang, M. et al. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood 109, 4280–4287 (2007).
Zimmermann, N. et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy 63, 1156–1163 (2008).
Song, D. J. et al. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin. Immunol. 131, 157–169 (2009).
Kiwamoto, T., Kawasaki, N., Paulson, J. C. & Bochner, B. S. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol. Ther. 135, 327–336 (2012).
Busse, W. et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J. Allergy Clin. Immunol. 108, 184–190 (2001).
Soler, M. et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur. Respir. J. 18, 254–261 (2001).
Holgate, S. T. et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin. Exp. Allergy 34, 632–638 (2004).
Holgate, S. T., Djukanovic, R., Casale, T. & Bousquet, J. Anti-immunoglobulin E treatment with omalizumab in allergic diseases: an update on anti-inflammatory activity and clinical efficacy. Clin. Exp. Allergy 35, 408–416 (2005).
Djukanovic, R. et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am. J. Respir. Crit. Care Med. 170, 583–593 (2004).
Massanari, M. et al. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Respir. Med. 104, 188–196 (2010).
Noga, O. et al. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. J. Allergy Clin. Immunol. 117, 1493–1499 (2006).
Foroughi, S. et al. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J. Allergy Clin. Immunol. 120, 594–601 (2007).
Ravetch, J. V. & Lanier, L. L. Immune inhibitory receptors. Science 290, 84–89 (2000).
Cooper, M. D. Inhibition of immune cell function. Immunol. Rev. 224, 7–10 (2008).
Munitz, A. & Levi-Schaffer, F. Inhibitory receptors on eosinophils: a direct hit to a possible Achilles heel? J. Allergy Clin. Immunol. 119, 1382–1387 (2007).
Munitz, A. Inhibitory receptors on myeloid cells: new targets for therapy? Pharmacol. Ther. 125, 128–137 (2010).
Munitz, A. et al. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood 107, 1996–2003 (2006).
Verjan, G. N. et al. SIRPα/CD172a regulates eosinophil homeostasis. J. Immunol. 187, 2268–2277 (2011).
Munitz, A., McBride, M. L., Bernstein, J. S. & Rothenberg, M. E. A dual activation and inhibition role for the paired immunoglobulin-like receptor B in eosinophils. Blood 111, 5694–5703 (2008).
Bachelet, I., Munitz, A. & Levi-Schaffer, F. Abrogation of allergic reactions by a bispecific antibody fragment linking IgE to CD300a. J. Allergy Clin. Immunol. 117, 1314–1320 (2006).
Park, L. S. et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 192, 659–670 (2000).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature Immunol. 3, 673–680 (2002).
Rimoldi, M. et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nature Immunol. 6, 507–514 (2005).
Zhang, Y. & Zhou, B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol. Res. 52, 211–223 (2012).
Osborn, M. J. et al. Overexpression of murine TSLP impairs lymphopoiesis and myelopoiesis. Blood 103, 843–851 (2004).
Wong, C. K., Hu, S., Cheung, P. F. & Lam, C. W. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am. J. Respir. Cell Mol. Biol. 43, 305–315 (2010).
Morshed, M., Yousefi, S., Stockle, C., Simon, H. U. & Simon, D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy 67, 1127–1137 (2012).
Gudbjartsson, D. F. et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nature Genet. 41, 342–347 (2009).
Rothenberg, M. E. et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nature Genet. 42, 289–291 (2010).
Klion, A. D. et al. Familial eosinophilia: a benign disorder? Blood 103, 4050–4055 (2004).
Bates, M. E. et al. Human airway eosinophils respond to chemoattractants with greater eosinophil-derived neurotoxin release, adherence to fibronectin, and activation of the Ras–ERK pathway when compared with blood eosinophils. J. Immunol. 184, 7125–7133 (2010).
Sedgwick, J. B. et al. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J. Immunol. 149, 3710–3718 (1992).
Coffer, P. J. & Koenderman, L. Granulocyte signal transduction and priming: cause without effect? Immunol. Lett. 57, 27–31 (1997).
Kariyawasam, H. H. & Robinson, D. S. The eosinophil: the cell and its weapons, the cytokines, its locations. Semin. Respir. Crit. Care Med. 27, 117–127 (2006).
Stein, M. L. et al. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J. Allergy Clin. Immunol. 121, 1473–1483.e4 (2008).
Pecaric-Petkovic, T., Didichenko, S. A., Kaempfer, S., Spiegl, N. & Dahinden, C. A. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood 113, 1526–1534 (2009).
Cherry, W. B., Yoon, J., Bartemes, K. R., Iijima, K. & Kita, H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J. Allergy Clin. Immunol. 121, 1484–1490 (2008).
Stolarski, B., Kurowska-Stolarska, M., Kewin, P., Xu, D. & Liew, F. Y. IL-33 exacerbates eosinophil-mediated airway inflammation. J. Immunol. 185, 3472–3480 (2010).
Kim, Y. H. et al. Anti-IL-33 antibody has a therapeutic effect in a murine model of allergic rhinitis. Allergy 67, 183–190 (2012).
Liu, X. et al. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem. Biophys. Res. Commun. 386, 181–185 (2009).
Yin, H. et al. Adenovirus-mediated delivery of soluble ST2 attenuates ovalbumin-induced allergic asthma in mice. Clin. Exp. Immunol. 170, 1–9 (2012).
Kang, J. H. et al. Regulation of functional phenotypes of cord blood derived eosinophils by γ-secretase inhibitor. Am. J. Respir. Cell Mol. Biol. 37, 571–577 (2007).
Radke, A. L. et al. Mature human eosinophils express functional Notch ligands mediating eosinophil autocrine regulation. Blood 113, 3092–3101 (2009).
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Eagar, T. N. et al. Notch 1 signaling regulates peripheral T cell activation. Immunity 20, 407–415 (2004).
Kang, J. H. et al. Eosinophilic differentiation is promoted by blockage of Notch signaling with a γ-secretase inhibitor. Eur. J. Immunol. 35, 2982–2990 (2005).
Siemers, E. R. et al. Effects of a γ-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology 66, 602–604 (2006).
Cameron, L. et al. Evidence for local eosinophil differentiation within allergic nasal mucosa: inhibition with soluble IL-5 receptor. J. Immunol. 164, 1538–1545 (2000).
Robinson, D. S. et al. CD34+/interleukin-5Rα messenger RNA+ cells in the bronchial mucosa in asthma: potential airway eosinophil progenitors. Am. J. Respir. Cell Mol. Biol. 20, 9–13 (1999).
Allakhverdi, Z. et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J. Allergy Clin. Immunol. 123, 472–478 (2009).
Menzies-Gow, A. et al. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J. Allergy Clin. Immunol. 111, 714–719 (2003).
Mejia, R. & Nutman, T. B. Evaluation and differential diagnosis of marked, persistent eosinophilia. Semin. Hematol. 49, 149–159 (2012).
Simon, H. U. et al. Refining the definition of hypereosinophilic syndrome. J. Allergy Clin. Immunol. 126, 45–49 (2010).
Caldwell, J. M. et al. Glucocorticoid-regulated genes in eosinophilic esophagitis: a role for FKBP51. J. Allergy Clin. Immunol. 125, 879–888 (2010).
Blanchard, C. et al. A striking local esophageal cytokine expression profile in eosinophilic esophagitis. J. Allergy Clin. Immunol. 127, 208–217 (2011).
Rothenberg, M. E. Biology and treatment of eosinophilic esophagitis. Gastroenterology 137, 1238–1249 (2009).
Dellon, E. S., Chen, X., Miller, C. R., Woosley, J. T. & Shaheen, N. J. Diagnostic utility of major basic protein, eotaxin-3, and leukotriene enzyme staining in eosinophilic esophagitis. Am. J. Gastroenterol. 107, 1503–1511 (2012).
Valent, P. et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J. Allergy Clin. Immunol. 130, 607–612 (2012).
Roufosse, F. & Weller, P. F. Practical approach to the patient with hypereosinophilia. J. Allergy Clin. Immunol. 126, 39–44 (2010).
Klion, A. D. How I treat hypereosinophilic syndromes. Blood 114, 3736–3741 (2009).
Acknowledgements
The authors thank S. Hottinger for editorial support and the following for support of their research programmes: the US National Institutes of Health (R01AI045898, R37AI045898, R01AI057803, R01AI061097, R01AI083450, R01DK076893, U01AI088806, U19AI070235, U19AI066738, P01HL076383 and K12HD028827); the US Department of Defense (W81XWH1010167); the CURED (Campaign Urging Research for Eosinophilic Disease) Foundation; the Buckeye Foundation; the Food Allergy Research & Education (FARE; formerly Food Allergy Initiative (FAI) and Food Allergy & Anaphylaxis Network (FAAN)); the Angels for Eosinophilic Research Alliance; and the Cincinnati Children's Hospital Medical Center.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
M.E.R. is a consultant for Immune Pharmaceuticals and has an equity interest, has a royalty interest for reslizumab (Teva Pharmaceuticals) and is an inventor of subject-related patents owned by Cincinnati Children's Hospital. P.C.F. declares no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Cincinnati Center for Eosinophilic Disorders website
Glossary
- Helminth infections
-
Infections with parasitic worms; helminth infections are the most common cause of eosinophilia worldwide, particularly in low-income countries. Infections with helminths can be transmitted via mosquito bites, by eating infected food, by drinking contaminated water and by walking on contaminated soil.
- Churg–Strauss syndrome
-
A rare systemic necrotizing vasculitis that affects small to medium-sized vessels and is characterized by asthma, blood eosinophilia and eosinophil-rich granulomatous inflammation in affected tissues. First-line treatment involves the use of corticosteroids.
- Hypereosinophilic syndrome
-
A heterogenous group of disorders characterized by a persistently elevated peripheral blood eosinophil count (> 1,500 eosinophils per mm3), typically without any recognizable cause.
- Eosinophilic oesophagitis
-
A chronic antigen-induced disease characterized by symptoms of oesophageal dysfunction, evidence of eosinophil infiltration of at least 15 eosinophils per high-power microscopy field on oesophageal biopsy and the exclusion of other possible causes of oesophageal eosinophilia, especially oesophageal eosinophilia induced by gastroesophageal reflux disease.
- Eotaxins
-
A subset of structurally related chemokines that bind to the eotaxin receptor (CC chemokine receptor 3) and are involved in selectively activating and chemoattracting eosinophils.
- Respiratory burst
-
The rapid release of destructive reactive oxygen species from eosinophils and other immune cells that have an important role in the immune response to infection; this contributes to oxidative damage during inflammatory responses.
- Atopic asthma
-
Also known as allergic asthma; a form of asthma that is characterized by recurrent attacks of difficulty in breathing with wheezing and inflammation and is caused by exposure to inhaled airborne allergens.
- Periostin
-
An extracellular matrix protein that interacts with integrin molecules on epithelial and leukocyte cell surfaces. Periostin expression is induced by T helper 2 (TH2) cytokines such as interleukin-4 (IL-4) and IL-13. Periostin promotes allergic inflammation, including the accumulation of eosinophils in the skin.
- Eosinopenia
-
A lower than expected number of eosinophils in the blood or tissue. Eosinopenia can be caused by stress reactions, bacterial infections and the use of corticosteroids.
- Antisense oligonucleotide
-
A synthesized strand of nucleic acid (DNA or RNA) that is complementary to a specific mRNA. Antisense oligonucleotides bind to their target mRNA to promote degradation of the mRNA and prevent its translation, which can ultimately lead to decreased expression of a particular protein.
- Splice-switching oligonucleotides
-
Oligonucleotides that target and bind to a particular mRNA and can be designed to promote favourable splice variants (rather than promoting degradation of the mRNA of targeted genes).
- IL-1RL1
-
Interleukin-1 receptor-like 1; also known as ST2 protein. A component of the receptor for IL-33 that is widely expressed by innate immune cells and a subset of T cell lymphocytes.
- Atopy
-
The predisposition to develop allergic hypersensitivity (that is, immunoglobulin E-mediated) reactions that can result from both hereditary and environmental components.
- FEV1
-
Forced expiratory volume in 1 second; the maximal amount of air that an individual can forcefully exhale in 1 second as calculated during pulmonary function testing. A normal FEV1 is predicted based on height, weight and race. A lower than normal FEV1 is a marker of an obstructive process such as asthma.
Rights and permissions
About this article
Cite this article
Fulkerson, P., Rothenberg, M. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov 12, 117–129 (2013). https://doi.org/10.1038/nrd3838
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3838
This article is cited by
-
Lignosus rhinocerotis extract ameliorates airway inflammation and remodelling via attenuation of TGF-β1 and Activin A in a prolonged induced allergic asthma model
Scientific Reports (2023)
-
N6-methyladenosine in myeloid cells: a novel regulatory factor for inflammation-related diseases
Journal of Physiology and Biochemistry (2023)
-
Osteoimmunology in Periodontitis and Orthodontic Tooth Movement
Current Osteoporosis Reports (2023)
-
Serum interleukin 38 (IL-38) as a new potential biomarker of pediatric asthma
The Egyptian Journal of Bronchology (2022)
-
Eosinophilic esophagitis auxiliary diagnosis based on a peptide ligand to eosinophil cationic protein in esophageal mucus of pediatric patients
Scientific Reports (2022)