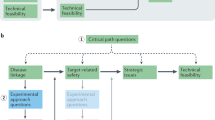
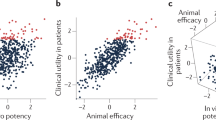
Abstract
Drug development projects have high attrition rates, often because efficacy and safety issues have not been foreseen. More effective prediction of 'translational success' could therefore have a key role in addressing the widely acknowledged problems with weak drug development pipelines. Here, I discuss how a scoring system to systematically assess key determinants of translational success, such as biomarkers and animal and human data, could help identify deficiencies and potential improvements, and increase the reliability of portfolio risk estimates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nature Rev. Drug Discov. 3, 711–715 (2004).
Nishimura, T. et al. Disease proteomics toward bedside reality. J. Gastroenterol. 40 S7–S13 (2005).
Wehling, M. Translational medicine: science or wishful thinking? J. Transl. Med. 6, 31–33 (2008).
Food and Drug Administration. Challenge and Opportunity on the Critical Path to New Medical Products. FDA website [online], (2004).
National Institutes of Health, Department of Health and Human Services. Clinical and Translational Science Awards. NIH website [online], (2006).
The European Advanced Translational Research Infrastructure in Medicine. Medical translation of basic research discoveries into clinical applications — a major challenge. EATRIS website [online], (2008).
Stroke Therapy Academic Industry Roundtable. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 30, 2752–2758 (1999).
Food and Drug Administration. Critical Path Opportunities Report. FDA website [online], (2006).
Burckart, G. J. et al. Qualification of biomarkers for drug development in organ transplantation. Am. J. Transplant. 8, 267–270 (2008).
European Medicines Agency. Medicines and Emerging Science. EMEA website [online], (2008).
The Biomarkers Consortium. Beta Cell Function Symposium. The Biomarkers Consortium website [online], (2009).
National Cancer Institute. Early Detection Reserach Network. NCI website [online], (2009).
Love, D. R. et al. Modeling inflammatory bowel disease: the zebrafish as a way forward. Expert Rev. Mol. Diagn. 7, 177–193 (2007).
Helgadottir, A. et al. A variant of the gene encoding leukotriene A4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nature Genet. 38, 68–74 (2006).
Jones, R., Armstrong, D., Malfertheiner, P. & Ducrotte, P. Does the treatment of gastroesophageal reflux disease (GERD) meet patients' needs? A survey-based study. Curr. Med. Res. Opin. 22, 657–662 (2006).
Coron, E., Hatlebakk, J. G. & Galmiche, J. P. Medical therapy of gastroesophageal reflux disease. Curr. Opin. Gastroenterol. 23, 434–439 (2007).
Koek, G. H. et al. Effect of the GABAB agonist baclofen in patients with symptoms and duodeno-gastro-oesophageal reflux refractory to proton pump inhibitors. Gut 52, 1397–1402 (2003).
Voshaar, T. et al. A randomized study of tiotropium Respimat Soft MistInhaler vs. ipratropium pMDI in COPD. Respir. Med. 102, 32–41 (2008).
Curry, S. H. Translational science: past, present, and future. Biotechniques 44, ii–viii (2008).
Wehling, M. Translational medicine: can it really facilitate the transition of research “from bench to bedside”? Eur. J. Clin. Pharmacol. 62, 91–95 (2006).
Wehling, M. Translational science in medicine — implications for the pharmaceutical industry. Int. J. Pharm. Med. 20, 303–310 (2006).
Schmidt, B. Proof of principle studies. Epilepsy Res. 68, 48–52 (2006).
Lipinski, C. A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 44, 235–249 (2000).
Izzo, J. L. Jr. Stagnation and the critical need for hypertension subtyping. J. Clin. Hypertens. (Greenwich). 10, 174–175 (2008).
Woo, S. & Jusko, W. J. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab. Dispos. 35, 1672–1678 (2007).
Kummar, S. et al. Phase 0 clinical trials: conceptions and misconceptions. Cancer J. 14, 133–137 (2008).
Mitcheson, J. S. hERG potassium channels and the structural basis of drug-induced arrhythmias. Chem. Res. Toxicol. 21, 1005–1010 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.W. was employed by AstraZeneca R&D, Molndal, as Director of Discovery Medicine (that is, translational medicine) from 2004 to 2006, while on sabbatical leave from his professorship at the University of Heidelberg. Having returned to this position in January 2007, he receives lecturing and consulting fees from Pfizer, Roche, Daiichi–Sankyo, Novartis, PAION and Lilly. He serves as a consultant to 4D Biomedical, Cambridge, UK, to promote translational assessment.
Related links
Related links
FURTHER INFORMATION
Early Detection Research Network, Biomarker Development Laboratories
Early Detection Research Network, Biomarker Reference Laboratories
Early Detection Research Network, Clinical Epidemiology and Validation Centers
Early Detection Research Network, Data Management and Coordinating Center
Rights and permissions
About this article
Cite this article
Wehling, M. Assessing the translatability of drug projects: what needs to be scored to predict success?. Nat Rev Drug Discov 8, 541–546 (2009). https://doi.org/10.1038/nrd2898
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd2898
This article is cited by
-
Translatability scoring in prospective and retrospective COVID drug development cases
European Journal of Clinical Pharmacology (2023)
-
Predictive validity in drug discovery: what it is, why it matters and how to improve it
Nature Reviews Drug Discovery (2022)
-
A Multi-Omics Interpretable Machine Learning Model Reveals Modes of Action of Small Molecules
Scientific Reports (2020)
-
Translatability score revisited: differentiation for distinct disease areas
Journal of Translational Medicine (2017)
-
Human genetics as a model for target validation: finding new therapies for diabetes
Diabetologia (2017)