Key Points
-
The concept of specific molecular targeting has been applied to the development of innovative cancer-treatment strategies. At present, two main approaches are available for use in clinical practice: therapeutic monoclonal antibodies (mAbs) and small-molecule agents.
-
We focus on the ErbB receptor family, particularly epidermal growth factor receptor (EGFR, also known as ERBB1) as an example of a target in our comparison of mAbs and small-molecule inhibitors. Cetuximab, a mAb, and gefitinib and erlotinib, which are small-molecule inhibitors, differ markedly in their basic properties and their underlying mechanisms of action.
-
The presence of activating mutations within the ATP-binding cleft of the EGFR kinase domain is associated with the sensitivity of non-small-cell lung cancer (NSCLC) to gefitinib, but not to cetuximab. By contrast, cetuximab shows a clinical benefit for colorectal cancers that overexpress EGFR in a manner independent of EGFR mutations. In malignant glioma, the sensitivity to gefitinib is closely related to deletions within the ectodomain of EGFR. In contrast to these drug-sensitivity mutations, the appearance of the T790M mutation confers resistance to gefitinib in NSCLC.
-
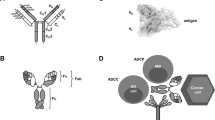
There are unique immune-effector mechanisms that are only triggered by therapeutic mAbs, such as antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity and complement-dependent cell-mediated cytotoxicity. By contrast, the effects of small-molecule agents are not directly linked to the activation of an immune response against tumour cells.
-
In general, mild adverse effects such as dermatological complications are commonly observed with these two classes of EGFR inhibitors. Although interstitial lung diseases or diarrhoea are more commonly associated with small-molecule therapies, therapeutic murine mAbs or chimeric mAbs can cause immunogenicity, leading to the production of human anti-mouse antibodies or human antichimeric antibodies, respectively.
-
It has been shown that mAbs such as trastuzumab and cetuximab exert synergistic anti-tumour effects in combination with chemotherapeutic agents more frequently than small-molecule inhibitors.
-
The combination of distinct classes of EGFR inhibitors could not only increase their efficacy, but also contribute to overcoming resistance to one class of EGFR inhibitor.
-
Further investigation into the distinct properties of these two classes of targeted agents should not only contribute to the development of new targeted agents but also provide an optimal therapeutic strategy for cancer treatment, thereby leading to the improvement of dual-targeted or multi-targeted therapy.
Abstract
The 'magic bullet' concept of specifically targeting cancer cells at the same time as sparing normal tissues is now proven, as several monoclonal antibodies and targeted small-molecule compounds have been approved for cancer treatment. Both antibodies and small-molecule compounds are therefore promising tools for target-protein-based cancer therapy. We discuss and compare the distinctive properties of these two therapeutic strategies so as to provide a better view for the development of new drugs and the future direction of cancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chabner, B. A. & Roberts, T. G., Jr. Timeline: chemotherapy and the war on cancer. Nature Rev. Cancer 5, 65–72 (2005).
Sawyers, C. Targeted cancer therapy. Nature 432, 294–297 (2004). A concise review of the molecular basis of targeted cancer therapy with a particular focus on protein kinase targets.
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Baselga, J. Targeting tyrosine kinases in cancer: the second wave. Science 312, 1175–1178 (2006). This is a very recent review of tyrosine kinase inhibitors in cancer therapy, which includes their brief histories and current issues that will affect the future development of new molecularly targeted agents.
Savage, D. G. & Antman, K. H. Imatinib mesylate-a new oral targeted therapy. N. Engl. J. Med. 346, 683–693 (2002).
Herbst, R. S., Fukuoka, M. & Baselga, J. Gefitinib-a novel targeted approach to treating cancer. Nature Rev. Cancer 4, 956–965 (2004). A comprehensive review of the EGFR inhibitor gefitinib, from the molecular mechanism of its inhibitory effects on EGFR signalling to its clinical development in NSCLC.
Carter, P. Improving the efficacy of antibody-based cancer therapies. Nature Rev. Cancer 1, 118–129 (2001).
Olayioye, M. A., Neve, R. M., Lane, H. A. & Hynes, N. E. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19, 3159–3167 (2000).
Yarden, Y. & Sliwkowski, M. X. Untangling the ErbB signalling network. Nature Rev. Mol. Cell Biol. 2, 127–137 (2001).
Mendelsohn, J. & Baselga, J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J. Clin. Oncol. 21, 2787–2799 (2003).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Green, L. L. et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nature Genet. 7, 13–21 (1994).
Maloney, D. G. et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90, 2188–2195 (1997).
Carter, P. et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc. Natl Acad. Sci. USA 89, 4285–4289 (1992).
Baselga, J., Norton, L., Albanell, J., Kim, Y. M. & Mendelsohn, J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 58, 2825–2831 (1998).
Krejsa, C., Rogge, M. & Sadee, W. Protein therapeutics: new applications for pharmacogenetics. Nature Rev. Drug Discov. 5, 507–521 (2006).
Molina, M. A. et al. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 61, 4744–4749 (2001).
Franklin, M. C. et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5, 317–328 (2004).
Agus, D. B. et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J. Clin. Oncol. 23, 2534–2543 (2005).
Ishida, T., Tsujisaki, M., Hinoda, Y., Imai, K. & Yachi, A. Establishment and characterization of mouse-human chimeric monoclonal antibody to erbB-2 product. Jpn. J. Cancer Res. 85, 172–178 (1994).
Hinoda, Y., Sasaki, S., Ishida, T. & Imai, K. Monoclonal antibodies as effective therapeutic agents for solid tumors. Cancer Sci. 95, 621–625 (2004).
Sasaki, S. et al. Human tumor growth suppression by apoptosis induced with anti-ErbB-2 chimeric monoclonal antibody. Jpn. J. Cancer Res. 89, 562–570 (1998).
Nahta, R., Hung, M. C. & Esteva, F. J. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 64, 2343–2346 (2004).
Huang, S. M. & Harari, P. M. Epidermal growth factor receptor inhibition in cancer therapy: biology, rationale and preliminary clinical results. Invest. New Drugs 17, 259–269 (1999).
Baselga, J. The EGFR as a target for anticancer therapy--focus on cetuximab. Eur. J. Cancer 37 (Suppl. 4), S16–S22 (2001).
Goldberg, R. M. Cetuximab. Nature Rev. Drug Discov. Suppl., S10–S11 (2005).
Gibson, T. B., Ranganathan, A. & Grothey, A. Randomized phase III trial results of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin. Colorectal Cancer 6, 29–31 (2006).
Pietras, R. J. et al. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 9, 1829–1838 (1994).
Izumi, Y., Xu, L., di Tomaso, E., Fukumura, D. & Jain, R. K. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature 416, 279–280 (2002).
Harding, J. & Burtness, B. Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody. Drugs Today (Barc.) 41, 107–127 (2005).
Maier, L. A. et al. Requirements for the internalization of a murine monoclonal antibody directed against the HER-2/neu gene product c-erbB-2. Cancer Res. 51, 5361–5369 (1991).
Brekke, O. H. & Sandlie, I. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nature Rev. Drug Discov. 2, 52–62 (2003). A review that describes the fundamental properties of antibodies and antibody engineering for their therapeutic applications, as well as antibody-mediated effector mechanisms such as ADCC or CDC.
Hudson, P. J. & Souriau, C. Engineered antibodies. Nature Med. 9, 129–134 (2003).
Arora, A. & Scholar, E. M. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharmacol. Exp. Ther. 315, 971–979 (2005).
Krause, D. S. & Van Etten, R. A. Tyrosine kinases as targets for cancer therapy. N. Engl. J. Med. 353, 172–187 (2005).
Druker, B. J. STI571 (Gleevec) as a paradigm for cancer therapy. Trends Mol. Med. 8, S14–S18 (2002).
Druker, B. J. Imatinib as a paradigm of targeted therapies. Adv. Cancer Res. 91, 1–30 (2004).
O'Dwyer, M. E., Mauro, M. J. & Druker, B. J. STI571 as a targeted therapy for CML. Cancer Invest. 21, 429–438 (2003).
Buchdunger, E., O'Reilly, T. & Wood, J. Pharmacology of imatinib (STI571). Eur. J. Cancer 38 Suppl. 5, S28–S36 (2002).
Mendelsohn, J. The epidermal growth factor receptor as a target for cancer therapy. Endocr. Relat. Cancer 8, 3–9 (2001).
Minna, J. D. & Dowell, J. Erlotinib hydrochloride. Nature Rev. Drug Discov. Suppl., S14–S15 (2005).
Chai, R. L. & Grandis, J. R. Advances in molecular diagnostics and therapeutics in head and neck cancer. Curr. Treat. Options Oncol. 7, 3–11 (2006).
Cohen, L. H. et al. Inhibitors of prenylation of Ras and other G-proteins and their application as therapeutics. Biochem. Pharmacol. 60, 1061–1068 (2000).
Sridhar, S. S., Hedley, D. & Siu, L. L. Raf kinase as a target for anticancer therapeutics. Mol. Cancer Ther. 4, 677–685 (2005).
Neckers, L. & Neckers, K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics- an update. Expert Opin. Emerg. Drugs 10, 137–149 (2005).
Sawyer, T. K. Cancer metastasis therapeutic targets and drug discovery: emerging small-molecule protein kinase inhibitors. Expert Opin. Investig. Drugs 13, 1–19 (2004).
Mannello, F., Tonti, G. & Papa, S. Matrix metalloproteinase inhibitors as anticancer therapeutics. Curr. Cancer Drug Targets 5, 285–298 (2005).
Marx, J. Cancer. Encouraging results for second-generation antiangiogenesis drugs. Science 308, 1248–1249 (2005).
Baker, M. Upping the ante on antibodies. Nature Biotechnol. 23, 1065–1072 (2005).
Reichert, J. M., Rosensweig, C. J., Faden, L. B. & Dewitz, M. C. Monoclonal antibody successes in the clinic. Nature Biotechnol. 23, 1073–1078 (2005).
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nature Rev. Drug Discov. 3, 711–715 (2004).
Butowski, N. & Chang, S. M. Small molecule and monoclonal antibody therapies in neurooncology. Cancer Control 12, 116–124 (2005).
Dancey, J. & Sausville, E. A. Issues and progress with protein kinase inhibitors for cancer treatment. Nature Rev. Drug Discov. 2, 296–313 (2003). A thorough review that gives an overview of the clinical development of various protein kinase inhibitors as targets of molecular-based cancer therapies.
Carter, P. J. Potent antibody therapeutics by design. Nature Rev. Immunol. 6, 343–357 (2006).
Li, S. et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 7, 301–311 (2005).
Cho, H. S. et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421, 756–760 (2003).
Huang, S., Armstrong, E. A., Benavente, S., Chinnaiyan, P. & Harari, P. M. Dual-agent molecular targeting of the epidermal growth factor receptor (EGFR): combining anti-EGFR antibody with tyrosine kinase inhibitor. Cancer Res. 64, 5355–5362 (2004).
Xia, W. et al. Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24, 6213–6221 (2005).
Matar, P. et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin. Cancer Res. 10, 6487–6501 (2004).
Thomas, S. M. & Grandis, J. R. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat. Rev. 30, 255–268 (2004).
Ranson, M. Epidermal growth factor receptor tyrosine kinase inhibitors. Br. J. Cancer 90, 2250–2255 (2004).
Rusnak, D. W. et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer Ther. 1, 85–94 (2001).
Geyer, C. E. et al. Scientific Special Session: Docetaxel added to Induction Therapy in Head and Neck Cancer. ASCO web site [online] (2006).
GlaxoSmithKline. GlaxoSmithKline receives positive data and halts enrolment in Phase III trial of Tykerb® (Lapatinib) in advanced breast cancer. GlaxoSmithKline web site [online] (2006).
Ravaud, A. et al. Efficacy of lapatinib in patients with high tumor EGFR expression: results of a phase III trial in advanced renal cell carcinoma (RCC). J. Clin. Oncol. 24, 4502 (2006).
Harrington, K. J. et al. A phase I, open-label study of lapatinib plus chemoradiation in patients with locally advanced squamous cell carcinoma of the head and neck (SCCHN). J. Clin. Oncol. 24, 5553 (2006).
Ciardiello, F. et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin. Cancer Res. 6, 2053–2063 (2000).
Moasser, M. M., Basso, A., Averbuch, S. D. & Rosen, N. The tyrosine kinase inhibitor ZD1839 ('Iressa') inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 61, 7184–7188 (2001).
Saltz, L. et al. Cetuximab (IMC-225) plus irinotecan (CPT-11) is active in CPT-11-refractory colorectal cancer that expresses epidermal growth factor receptors. Proc. Am. Soc. Clin. Oncol. 20, 3 (2001).
Bailey, L. R. et al. Tumor EGFR membrane staining is not clinically relevant for predicting response in patients receiving gefitinib ('Iressa', ZD1839) monotherapy for pretreated advanced non-small-cell lung cancer: IDEAL 1 and 2. Proc. Am. Assoc. Cancer Res. 44, 1362 (2003).
Shepherd, F. A. et al. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 353, 123–132 (2005).
Arteaga, C. L. & Baselga, J. Clinical trial design and end points for epidermal growth factor receptor-targeted therapies: implications for drug development and practice. Clin. Cancer Res. 9, 1579–1589 (2003).
Bianco, R., Troiani, T., Tortora, G. & Ciardiello, F. Intrinsic and acquired resistance to EGFR inhibitors in human cancer therapy. Endocr. Relat. Cancer 12 Suppl. 1, S159–S171 (2005).
Bishop, P. C. et al. Differential sensitivity of cancer cells to inhibitors of the epidermal growth factor receptor family. Oncogene 21, 119–127 (2002).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004). The above two reports (references 75 and 76) show the identification of somatic mutations in exons 18–21 of the EGFR gene in NSCLC. These results indicated these EGFR mutations as possible determinants of gefitinib sensitivity to NSCLC.
Han, S. W. et al. Predictive and prognostic impact of epidermal growth factor receptor mutation in non-small-cell lung cancer patients treated with gefitinib. J. Clin. Oncol. 23, 2493–2501 (2005).
Huang, S. F. et al. High frequency of epidermal growth factor receptor mutations with complex patterns in non-small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin. Cancer Res. 10, 8195–8203 (2004).
Kosaka, T. et al. Mutations of the epidermal growth factor receptor gene in lung cancer: biological and clinical implications. Cancer Res. 64, 8919–8923 (2004).
Miller, V. A. et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J. Clin. Oncol. 22, 1103–1109 (2004).
Pao, W. et al. EGF receptor gene mutations are common in lung cancers from 'never smokers' and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl Acad. Sci. USA 101, 13306–13311 (2004).
Shigematsu, H. et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl Cancer Inst. 97, 339–346 (2005).
Tracy, S. et al. Gefitinib induces apoptosis in the EGFRL858R non-small-cell lung cancer cell line H3255. Cancer Res. 64, 7241–7244 (2004).
Cappuzzo, F. et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J. Natl Cancer. Inst. 97, 643–655 (2005).
Cappuzzo, F. et al. Increased HER2 gene copy number is associated with response to gefitinib therapy in epidermal growth factor receptor-positive non-small-cell lung cancer patients. J. Clin. Oncol. 23, 5007–5018 (2005).
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005). This paper documented the identification of a second mutation (T790M) in NSCLC bearing an activating EGFR mutation, which might be related to the resistance of NSCLC to gefitinib.
Frederick, L., Wang, X. Y., Eley, G. & James, C. D. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 60, 1383–1387 (2000).
Mellinghoff, I. K. et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 (2005).
Mukohara, T. et al. Differential effects of gefitinib and cetuximab on non-small-cell lung cancers bearing epidermal growth factor receptor mutations. J. Natl Cancer Inst. 97, 1185–1194 (2005). An interesting report of a comparative evaluation of the sensitivites of gefitinib and cetuximab to NSCLC cell lines that habour EGFR mutations, and consistent data by retrospective analysis of NSCLC patients with EGFR mutations treated with both gefitinib and cetuximab.
Amann, J. et al. Aberrant epidermal growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 65, 226–235 (2005).
Chung, K. Y. et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J. Clin. Oncol. 23, 1803–1810 (2005).
Cunningham, D. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345 (2004).
Mackenzie, M. J. et al. A phase II trial of ZD1839 (Iressa) 750 mg per day, an oral epidermal growth factor receptor-tyrosine kinase inhibitor, in patients with metastatic colorectal cancer. Invest. New Drugs 23, 165–170 (2005).
Saltz, L. B. et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J. Clin. Oncol. 22, 1201–1208 (2004).
Ogino, S. et al. Molecular alterations in tumors and response to combination chemotherapy with gefitinib for advanced colorectal cancer. Clin. Cancer Res. 11, 6650–6656 (2005).
Italiano, A. Targeting the epidermal growth factor receptor in colorectal cancer: advances and controversies. Oncology 70, 161–167 (2006).
Moroni, M. et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 6, 279–286 (2005).
Bianco, R. et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene 22, 2812–2822 (2003).
She, Q. B., Solit, D., Basso, A. & Moasser, M. M. Resistance to gefitinib in PTEN-null HER-overexpressing tumor cells can be overcome through restoration of PTEN function or pharmacologic modulation of constitutive phosphatidylinositol 3'-kinase/Akt pathway signaling. Clin. Cancer Res. 9, 4340–4346 (2003).
Sordella, R., Bell, D. W., Haber, D. A. & Settleman, J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305, 1163–1167 (2004). Describes the essential role of AKT and STAT signalling pathways in mutant EGFR-mediated cell survival, which provide a putative mechanism underlying the therapeutic effect of gefitinib in NSCLC.
Janmaat, M. L., Kruyt, F. A., Rodriguez, J. A. & Giaccone, G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin. Cancer Res. 9, 2316–2326 (2003).
Li, B., Chang, C. M., Yuan, M., McKenna, W. G. & Shu, H. K. Resistance to small molecule inhibitors of epidermal growth factor receptor in malignant gliomas. Cancer Res. 63, 7443–7450 (2003).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Dean, M., Fojo, T. & Bates, S. Tumour stem cells and drug resistance. Na ture Rev. Cancer 5, 275–284 (2005).
Michor, F. et al. Dynamics of chronic myeloid leukaemia. Nature 435, 1267–1270 (2005).
Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature Med. 6, 443–446 (2000).
Iannello, A. & Ahmad, A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 24, 487–499 (2005).
Cartron, G. et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99, 754–758 (2002).
Weng, W. K. & Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003).
Fan, Z., Masui, H., Altas, I. & Mendelsohn, J. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 53, 4322–4328 (1993).
Hale, G., Clark, M. & Waldmann, H. Therapeutic potential of rat monoclonal antibodies: isotype specificity of antibody-dependent cell-mediated cytotoxicity with human lymphocytes. J. Immunol. 134, 3056–3061 (1985).
Weng, W. K. & Levy, R. Expression of complement inhibitors CD46, CD55, and CD59 on tumor cells does not predict clinical outcome after rituximab treatment in follicular non-Hodgkin lymphoma. Blood 98, 1352–1357 (2001).
Manches, O. et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood 101, 949–954 (2003).
Di Gaetano, N. et al. Complement activation determines the therapeutic activity of rituximab in vivo. J. Immunol. 171, 1581–1587 (2003).
Cragg, M. S. & Glennie, M. J. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood 103, 2738–2743 (2004).
Chan, H. T. et al. CD20-induced lymphoma cell death is independent of both caspases and its redistribution into triton X-100 insoluble membrane rafts. Cancer Res. 63, 5480–5489 (2003).
Cragg, M. S. et al. Complement-mediated lysis by anti-CD20 mAb correlates with segregation into lipid rafts. Blood 101, 1045–1052 (2003).
Gorter, A. & Meri, S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol. Today 20, 576–582 (1999).
Lazar, G. A. et al. Engineered antibody Fc variants with enhanced effector function. Proc. Natl Acad. Sci. USA 103, 4005–4010 (2006).
Li, H. et al. Optimization of humanized IgGs in glycoengineered Pichia pastoris. Nature Biotechnol. 24, 210–215 (2006).
Umana, P., Jean-Mairet, J., Moudry, R., Amstutz, H. & Bailey, J. E. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nature Biotechnol. 17, 176–180 (1999).
Teeling, J. L. et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 104, 1793–1800 (2004).
Idusogie, E. E. et al. Engineered antibodies with increased activity to recruit complement. J. Immunol. 166, 2571–2575 (2001).
Dancey, J. E. & Freidlin, B. Targeting epidermal growth factor receptor — are we missing the mark? Lancet 362, 62–64 (2003).
Herbst, R. S., LoRusso, P. M., Purdom, M. & Ward, D. Dermatologic side effects associated with gefitinib therapy: clinical experience and management. Clin. Lung Cancer 4, 366–369 (2003).
Perez-Soler, R. & Saltz, L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J. Clin. Oncol. 23, 5235–5246 (2005). This review describes the association between EGFR-targeted agents and skin rash, a common adverse effect, and considers the possibility that this observation could be an indicator of the efficacy of EGFR inhibition.
Buter, J. & Giaccone, G. Medical treatment of non-small-cell lung cancer. Ann. Oncol. 16 Suppl. 2, ii229–ii232 (2005).
Thatcher, N. et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366, 1527–1537 (2005).
Baselga, J. et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J. Clin. Oncol. 18, 904–914 (2000).
Inoue, A. et al. Severe acute interstitial pneumonia and gefitinib. Lancet 361, 137–139 (2003).
Endo, M., Johkoh, T., Kimura, K. & Yamamoto, N. Imaging of gefitinib-related interstitial lung disease: multi-institutional analysis by the West Japan Thoracic Oncology Group. Lung Cancer 52, 135–140 (2006).
Calvo, E. & Baselga, J. Ethnic differences in response to epidermal growth factor receptor tyrosine kinase inhibitors. J. Clin. Oncol. 24, 2158–2163 (2006).
Elkind, N. B. et al. Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib). Cancer Res. 65, 1770–1777 (2005).
Khazaeli, A. L., Falcey, J., Paulter, V., Fetzer, M. & Waksal, H. Low immunogenicity of a chimeric monoclonal antibody (MoAb), IMC-C225, used to treat epidermal growth factor receptor-positive tumors. Proc. Am. Soc. Clin. Oncol. abstr 808 (2000).
Fukuoka, M. et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J. Clin. Oncol. 21, 2237–2246 (2003).
Kris, M. G. et al. A phase II trial of ZD1839 (Iressa) in advanced non-small cell lung cancer (NSCLC) patients who had failed platinum- and docetaxel-based regimens (IDEAL 2). Proc. Am. Soc. Clin. Oncol. 21, 292a (2002).
Perez-Soler, R. et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J. Clin. Oncol. 22, 3238–3247 (2004).
Shepherd, F. A., Pereira, J. & Ciuleanu, T. E. A randomized placebo-controlled trial of erlotinib in patients with advanced non-small cell lung cancer (NSCLC) following failure of 1st line or 2nd line chemotherapy: A National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) trial. J. Clin. Oncol. 22, 622s (2004).
Mendelsohn, J. Epidermal growth factor receptor inhibition by a monoclonal antibody as anticancer therapy. Clin. Cancer Res. 3, 2703–2707 (1997).
Lilenbaum, R. et al. A phase II trial of cetuximab as therapy for recurrent non-small cell lung cancer (NSCLC). ASCO Meeting Proc. 23, 7036 (2005).
Giaccone, G. et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial — INTACT 1. J. Clin. Oncol. 22, 777–784 (2004).
Herbst, R. S. et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial — INTACT 2. J. Clin. Oncol. 22, 785–794 (2004).
Rosell, R. et al. Randomized phase II study of cetuximab in combination with cisplatin (C) and vinorelbine (V) vs. CV alone in the first-line treatment of patients (pts) with epidermal growth factor receptor (EGFR)-expressing advanced non-small-cell lung cancer (NSCLC). J. Clin. Oncol. 22, 618s (2004).
Thienelt, C. D. et al. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancer. J. Clin. Oncol. 23, 8786–8793 (2005).
Wong, S. F. Cetuximab: an epidermal growth factor receptor monoclonal antibody for the treatment of colorectal cancer. Clin. Ther. 27, 684–694 (2005).
Kuo, T. & Fisher, G. A. Current status of small-molecule tyrosine kinase inhibitors targeting epidermal growth factor receptor in colorectal cancer. Clin. Colorectal Cancer 5 Suppl. 2, S62–S70 (2005).
Bonner, J. A., & Harari, M. Cetuximab prolongs survival in patients with locoregionally advanced squamous cell carcinoma of head and neck: A phase III study of high dose radiation therapy with or without cetuximab. J. Clin. Oncol. 22, 489s (2004).
Doss, H. H. et al. Induction chemotherapy + gefitinib followed by concurrent chemotherapy/radiation therapy/gefitinib for patients (pts) with locally advanced squamous carcinoma of the head and neck: a phase I/II trial of the Minnie Pearl Cancer Research Network. J. Clin. Oncol. 24, 5543 (2006).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Lowe, S. W., Ruley, H. E., Jacks, T. & Housman, D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74, 957–967 (1993).
Takaoka, A. et al. Integration of interferon-a/b signalling to p53 responses in tumour suppression and antiviral defence. Nature 424, 516–523 (2003).
Huether, A., Hopfner, M., Baradari, V., Schuppan, D. & Scherubl, H. EGFR blockade by cetuximab alone or as combination therapy for growth control of hepatocellular cancer. Biochem. Pharmacol. 70, 1568–1578 (2005).
Ince, W. L. et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J. Natl Cancer Inst. 97, 981–989 (2005).
Minna, J. D., Peyton, M. J. & Gazdar, A. F. Gefitinib versus cetuximab in lung cancer: round one. J. Natl Cancer Inst. 97, 1168–1169 (2005).
Xia, W., Liu, L. H., Ho, P. & Spector, N. L. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene 23, 646–653 (2004).
Konecny, G. E. et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 66, 1630–1639 (2006).
Storniolo, A. et al. A phase I, open-label study of lapatinib (GW572016) plus trastuzumab; a clinically active regimen. J. Clin. Oncol. 23, 559 (2005).
Williams, M. Target validation. Curr. Opin. Pharmacol. 3, 571–577 (2003).
Knight, Z. A. et al. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell 125, 733–747 (2006).
Raben, D. et al. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin. Cancer Res. 11, 795–805 (2005).
Yilmaz, O. H. et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441, 475–482 (2006).
Zhang, J. et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441, 518–522 (2006).
Fabian, M. A. et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nature Biotechnol. 23, 329–336 (2005).
Acknowledgements
We would like to thank T. Ishida for his continuous support of the work in our laboratory described in this Review. The work in our laboratory was supported in part by a grant for Advanced Research on Cancer from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We also thank Z. Wang for his assistance with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Glossary
- Bacteriophage display
-
A display method for identifying proteins or peptides that recognize and bind to a target molecule(s). Bacteriophages that display the antibody of interest are selected by antigen binding and are propagated in bacteria. This helps identify therapeutic antibodies with high binding affinity.
- Shedding
-
The release of the extracellular domain of a cell-membrane protein, such as a growth-factor receptor, from the cell surface. ERBB2 is proteolytically cleaved, possibly by a matrix metalloproteinase activator, although this proteolysis does not seem to be mediated by a general shedding system that can be activated by protein kinase C. ERBB2 cleavage generates a membrane-associated receptor fragment with potentially increased tyrosine kinase activity.
- Complement-dependent cytotoxicity
-
This is one of the antigen-elimination processes that is mediated by immunoglobulins (Ig). When IgM and certain IgG subclasses (IgG1 and IgG3) bind to an antigen, one of the complement factors is strongly activated. Then, a sequence of cleavage reactions of other complement factors (classical pathway of complement activation) is triggered to activate their cytotoxic function, which leads to the destruction of the target cells.
- Antibody-dependent cellular cytotoxicity
-
This reaction can be initiated by the Fc portion of immunoglobulins (Ig). Phagocytes such as monocytes/macrophages, dendritic cells, natural killer cells and neutrophils take up IgG-coated target cells through binding with Fcγ-receptors on the surface of the phagocytes. This is eventually followed by the elimination of target cells.
- ATP mimetics
-
These small-molecule inhibitors competitively bind to the ATP-binding cleft at the activation loop of target kinases, thereby inhibiting their kinase activity.
- Chymotryptic protease in the 26S proteasome
-
The 26S proteasome is a multicatalytic complex, which is composed of the 20S catalytic core subunit and the 19S regulatory subunit that recognize and degrade ubiquitylated proteins. A chymotrypsin-like proteolytic activity is one of the catalytic activities of this core subunit for the hydrolysis of peptide substrates.
- Complement-dependent cellular cytotoxicity
-
This is a cell-mediated effector mechanism for target cell killing. As similarly observed in CDC, complement activation is triggered in CDCC by the interaction of C1 q to the Fc regions of antibodies bound to target antigens. During this process, several complement components, such as C3b, are generated and recognized by effector immune cells through their complementary receptors, which leads to phagocytosis and cytotoxicity.
- Opsonins
-
Opsonins are any molecules with which antigens are coated, such as IgG and components of complement factors (C1 q, C3b, iC3b, and C4b), to become more susceptible to phagocytosis by macrophages or neutrophils.These phagocytes bind opsonin molecules through Fcγ receptors or complement receptors that are expressed on their surface membrane.
- Cancer stem cells
-
A small subpopulation of quiescent tumour cells within a tumour that have properties similar to normal stem cells, such as the capability to undergo self-renewal and to maintain tumour growth and heterogeneity. According to the stem-cell-based model, conventional therapies typically target actively proliferating cells but spare drug-resistant cancer stem cells, which might contribute to therapeutic failure and eventual relapses.
- Pruritus
-
A dermatological symptom (itching) that is often observed in cutaneous lesions caused by allergy and infections.
- Asthenia
-
A general feeling of weakness or lack of vigour, which can be associated with various diseases.
- Anaphylactoid reactions
-
Systemic immunological hyper-responses that mimic anaphylaxis. In contrast to IgE-mediated anaphylactic reactions, these are triggered by an IgE-independent mechanism, frequently appear as allergic reactions to drugs, foods and exercise, and manifest as potentially life-threatening symptoms such as hypotension, bronchospasm and laryngeal oedema.
- Urticaria
-
A cutaneous symptom that primarily manifests as a rash and pruritus. This manifestation is caused by IgE- or non-IgE hypersensitivity with histamine and other vasoactive chemicals released from mast cells as a result of exposure to drugs and foods.
- Interstitial pneumonitis
-
A form of pneumonia that is characterized by non-infectious inflammation and fibrosis in the space between the epithelial and endothelial basement membranes of the lower respiratory tract. This is caused by unknown and known factors such as drugs (gefitinib, lefluomide or irinotecan) or environmental factors, and can be observed in association with other diseases (for example, connective tissue diseases). Patients with this disorder typically present with cough and shortness of breath.
- Human anti-mouse antibodies
-
HAMAs are antibodies that are produced by the human immune system against therapeutic murine monoclonal antibodies (mAbs)
- Human anti-chimeric antibodies
-
HACAs are antibodies that are produced against murine components of chimeric or humanized mAbs. HAMAs and HACAs are often related to immunogenicity problems associated with a lack of efficacy and rapid clearance during mAb therapy.
Rights and permissions
About this article
Cite this article
Imai, K., Takaoka, A. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 6, 714–727 (2006). https://doi.org/10.1038/nrc1913
Issue Date:
DOI: https://doi.org/10.1038/nrc1913
This article is cited by
-
Therapeutic antibodies for the prevention and treatment of cancer
Journal of Biomedical Science (2024)
-
Steroid Premedication and Monoclonal Antibody Therapy: Should We Reconsider?
Current Treatment Options in Oncology (2024)
-
A MIST conception: what has been learned from twenty years of human metabolite safety assessment?
Medicinal Chemistry Research (2023)
-
Peptide-Based Therapeutics in Cancer Therapy
Molecular Biotechnology (2023)
-
Novel biomolecules in targeted cancer therapy: a new approach towards precision medicine
Medical Oncology (2023)