Key Points
-
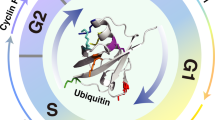
Two major classes of ubiquitin ligases, the SKP1–CUL1–F-box-protein (SCF) complex and the anaphase-promoting complex/cyclosome (APC/C), have a central role in cell-cycle regulation.
-
The SCF complex and APC/C are structurally similar. Each is constituted of common subunits and a variable substrate-recognition subunit (F-box proteins for the SCF complex and activators for the APC/C). Three F-box proteins in the SCF complex — S-phase kinase-associated protein 2 (SKP2), F-box and WD-40 domain protein 7 (FBW7) and β-transducin repeat-containing protein (β-TRCP) — and two activators in the APC/C — cell division cycle 20 (CDC20) and CDH1 (also known as HCT1) — are the most important in cell-cycle regulation.
-
SKP2 targets negative regulators of the cell cycle such as p27, p21 and p57 for degradation, and thereby promotes cell-cycle progression during S and G2 phases. SKP2 is upregulated in many human cancers.
-
FBW7 induces the degradation of positive regulators of the cell cycle, such as MYC, JUN, cyclin E and Notch. FBW7 is often mutated in a subset of human cancers.
-
β-TRCP is a versatile F-box protein that recognizes several cell-cycle regulators — EMI1/2, WEE1A and CDC25A/B — in addition to its classical substrates, β-catenin and IκB. In some cancers, β-TRCP mutation or overexpression is found.
-
CDC20 targets securin and mitotic cyclins for destruction, and thereby promotes sister-chromatid separation. CDC20 is the crucial mediator of the spindle checkpoint, which prevents aneuploidy and genomic instability. CDC20 is overexpressed in some cancers, although in others the CDC20 gene is mutated or deleted.
-
CDH1 facilitates exit from M phase and maintains G1 phase by mediating the degradation of mitotic cyclins, non-CDK (cyclin-dependent kinase) mitotic kinases and some regulators of the formation of pre-replicative complexes. Deregulated expression or mutation of CDH1 as well as of most CDH1 targets have been described in human cancers.
Abstract
A driving force of the cell cycle is the activation of cyclin-dependent kinases (CDKs), the activities of which are controlled by the ubiquitin-mediated proteolysis of key regulators such as cyclins and CDK inhibitors. Two ubiquitin ligases, the SKP1–CUL1–F-box-protein (SCF) complex and the anaphase-promoting complex/cyclosome (APC/C), are responsible for the specific ubiquitylation of many of these regulators. Deregulation of the proteolytic system might result in uncontrolled proliferation, genomic instability and cancer. Cumulative clinical evidence shows alterations in the ubiquitylation of cell-cycle regulators in the aetiology of many human malignancies. A better understanding of the ubiquitylation machinery will provide new insights into the regulatory biology of cell-cycle transitions and the development of anti-cancer drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Evans, T., Rosenthal, E. T., Youngblom, J., Distel, D. & Hunt, T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell 33, 389–396 (1983).
Glotzer, M., Murray, A. W. & Kirschner, M. W. Cyclin is degraded by the ubiquitin pathway. Nature 349, 132–138 (1991). Shows that cyclins are degraded by the ubiquitin-proteasome pathway and defines the D-box degron.
Hershko, A. Ubiquitin: roles in protein modification and breakdown. Cell 34, 11–12 (1983).
Okamoto, Y. et al. UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Res. 63, 4167–4173 (2003).
Bloom, J. & Pagano, M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin. Cancer Biol. 13, 41–47 (2003).
Pagano, M. et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269, 682–685 (1995). The first paper describing ubiquitylation of p27.
Shirane, M. et al. Down-regulation of p27Kip1 by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J. Biol. Chem. 274, 13886–13893 (1999).
Nakayama, K. I. & Nakayama, K. Regulation of the cell cycle by SCF-type ubiquitin ligases. Semin. Cell Dev. Biol. 16, 323–333 (2005).
Harper, J. W., Burton, J. L. & Solomon, M. J. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16, 2179–2206 (2002).
Castro, A., Bernis, C., Vigneron, S., Labbe, J. C. & Lorca, T. The anaphase-promoting complex: a key factor in the regulation of cell cycle. Oncogene 24, 314–325 (2005).
Jin, J. et al. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 18, 2573–2580 (2004).
Aulia, S. & Tang, B. L. Cdh1-APC/C, cyclin B-Cdc2, and Alzheimer's disease pathology. Biochem. Biophys. Res. Commun. 339, 1–6 (2006).
Guardavaccaro, D. et al. Control of meiotic and mitotic progression by the F box protein β-Trcp1 in vivo. Dev. Cell 4, 799–812 (2003).
Margottin-Goguet, F. et al. Prophase destruction of Emi1 by the SCFβTrCP/Slimb ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4, 813–826 (2003). References 13 and 14 show the interplay between the SCF complex and the APC/C through the degradation of EMI1, an inhibitor of APC/C, by SCFβ–TRCP.
Bashir, T., Dorrello, N. V., Amador, V., Guardavaccaro, D. & Pagano, M. Control of the SCFSkp2–Cks1 ubiquitin ligase by the APC/CCdh1 ubiquitin ligase. Nature 428, 190–193 (2004).
Wei, W. et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428, 194–198 (2004). References 15 and 16 show the interplay between the SCF complex and APC/C through the degradation of SKP2 by APC/CCDH1.
Zhang, H., Kobayashi, R., Galaktionov, K. & Beach, D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell 82, 915–925. (1995).
Carrano, A. C., Eytan, E., Hershko, A. & Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biol. 1, 193–199 (1999).
Sutterluty, H. et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nature Cell Biol. 1, 207–214 (1999).
Tsvetkov, L. M., Yeh, K. H., Lee, S. J., Sun, H. & Zhang, H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr. Biol. 9, 661–664 (1999). References 18–20 show that SKP2 targets p27 for degradation only when it is phosphorylated on Thr187.
Yu, Z. K., Gervais, J. L. & Zhang, H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proc. Natl Acad. Sci. USA 95, 11324–11329 (1998).
Bornstein, G. et al. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 278, 25752–25757 (2003).
Kamura, T. et al. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc. Natl Acad. Sci. USA 100, 10231–10236 (2003).
Nakayama, K. et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 (2000). Describes Skp2-deficient mice, which show over-replication problems with a marked accumulation of p27.
Nakayama, K. et al. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell 6, 661–672 (2004). Shows that p27 is one of the main substrates of SCFSKP2, as the SKP2−/− phenotype is rescued by p27 deficiency. It also shows that p27 inhibits CDK1 as well as CDK2.
Kossatz, U. et al. Skp2-dependent degradation of p27kip1 is essential for cell cycle progression. Genes Dev. 18, 2602–2607 (2004).
Fero, M. L. et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 85, 733–744 (1996).
Kiyokawa, H. et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell 85, 721–732 (1996).
Nakayama, K. et al. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85, 707–720 (1996).
Yokoi, S. et al. A novel target gene, SKP2, within the 5p13 amplicon that is frequently detected in small cell lung cancers. Am. J. Pathol. 161, 207–216 (2002).
Yokoi, S. et al. Amplification and overexpression of SKP2 are associated with metastasis of non-small-cell lung cancers to lymph nodes. Am. J. Pathol. 165, 175–180 (2004).
Dowen, S. E. et al. Amplification of chromosome 5p correlates with increased expression of Skp2 in HPV-immortalized keratinocytes. Oncogene 22, 2531–2540 (2003).
Shapira, M. et al. Alterations in the expression of the cell cycle regulatory protein cyclin kinase subunit 1 in colorectal carcinoma. Cancer 100, 1615–1621 (2004).
Shapira, M. et al. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer 103, 1336–1346 (2005).
Latres, E. et al. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl Acad. Sci. USA 98, 2515–2520 (2001).
Shim, E. H. et al. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 63, 1583–1588 (2003).
Imaki, H. et al. Cell cycle-dependent regulation of the Skp2 promoter by GA-binding protein. Cancer Res. 63, 4607–4613 (2003).
Hara, T. et al. Degradation of p27Kip1 at the G0-G1 transition mediated by a Skp2-independent ubiquitination pathway. J. Biol. Chem. 276, 48937–48943 (2001).
Ishida, N. et al. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 277, 14355–14358 (2002).
Rodier, G. et al. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 20, 6672–6682 (2001).
Miura, M., Hatakeyama, S., Hattori, K. & Nakayama, K. I. Structure and expression of the gene encoding mouse F-box protein, Fwd2. Genomics 62, 50–58 (1999).
Kamura, T. et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27Kip1 at G1 phase. Nature Cell Biol. 6, 1229–1235 (2004). Identifies the second E3 ligase for p27 that functions during G1 phase in the cytoplasm.
Kotoshiba, S., Kamura, T., Hara, T., Ishida, N. & Nakayama, K. I. Molecular dissection of the interaction between p27 and Kip1 ubiquitylation-promoting complex, the ubiquitin ligase that regulates proteolysis of p27 in G1 phase. J. Biol. Chem. 280, 17694–17700 (2005).
Hara, T. et al. Role of the UBL-UBA protein KPC2 in degradation of p27 at G1 phase of the cell cycle. Mol. Cell. Biol. 25, 9292–9303 (2005).
Signoretti, S. et al. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J. Clin. Invest. 110, 633–641 (2002).
Hubbard, E. J., Wu, G., Kitajewski, J. & Greenwald, I. sel-10, a negative regulator of lin-12 activity in Caenorhabditis elegans, encodes a member of the CDC4 family of proteins. Genes Dev. 11, 3182–3193 (1997).
Tsunematsu, R. et al. Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J. Biol. Chem. 279, 9417–9423 (2004).
Tetzlaff, M. T. et al. Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc. Natl Acad. Sci. USA 101, 3338–3345 (2004).
Mao, J. H. et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature 432, 775–779 (2004).
Spruck, C. H., Won, K. A. & Reed, S. I. Deregulated cyclin E induces chromosome instability. Nature 401, 297–300 (1999).
Rajagopalan, H. et al. Inactivation of hCDC4 can cause chromosomal instability. Nature 428, 77–81 (2004).
Ekholm-Reed, S. et al. Mutation of hCDC4 leads to cell cycle deregulation of cyclin E in cancer. Cancer Res. 64, 795–800 (2004).
Adhikary, S. & Eilers, M. Transcriptional regulation and transformation by Myc proteins. Nature Rev. Mol. Cell Biol. 6, 635–645 (2005).
Yada, M. et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 23, 2116–2125 (2004).
Welcker, M. et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl Acad. Sci. USA 101, 9085–9090 (2004).
Kim, S. Y., Herbst, A., Tworkowski, K. A., Salghetti, S. E. & Tansey, W. P. Skp2 regulates Myc protein stability and activity. Mol. Cell 11, 1177–1188 (2003).
von der Lehr, N. et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell 11, 1189–1200 (2003). References 54 and 55 show that FBW7 targets MYC for degradation only when MB1 is phosphorylated, while references 56 and 57 show that SKP2 binds to, and activates, MYC.
Weng, A. P. & Aster, J. C. Multiple niches for Notch in cancer: context is everything. Curr. Opin. Genet. Dev. 14, 48–54 (2004).
Capobianco, A. J., Zagouras, P., Blaumueller, C. M., Artavanis-Tsakonas, S. & Bishop, J. M. Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol. Cell. Biol. 17, 6265–6273 (1997).
Pear, W. S. et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J. Exp. Med. 183, 2283–2291 (1996).
Callahan, R. & Raafat, A. Notch signaling in mammary gland tumorigenesis. J. Mammary Gland Biol. Neoplasia 6, 23–36 (2001).
Hoemann, C. D., Beaulieu, N., Girard, L., Rebai, N. & Jolicoeur, P. Two distinct Notch1 mutant alleles are involved in the induction of T-cell leukemia in c-myc transgenic mice. Mol. Cell. Biol. 20, 3831–3842 (2000).
Weijzen, S. et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nature Med. 8, 979–986 (2002).
Gallahan, D. & Callahan, R. The mouse mammary tumor associated gene INT3 is a unique member of the NOTCH gene family (NOTCH4). Oncogene 14, 1883–1890 (1997).
Hartl, M., Bader, A. G. & Bister, K. Molecular targets of the oncogenic transcription factor jun. Curr. Cancer Drug Targets 3, 41–55 (2003).
Tung, J. J. et al. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl Acad. Sci. USA 102, 4318–4323 (2005).
Schmidt, A. et al. Xenopus polo-like kinase Plx1 regulates XErp1, a novel inhibitor of APC/C activity. Genes Dev. 19, 502–513 (2005). References 66 and 67 show that EMI2, an inhibitor of the APC/C, is essential for the CSF-arrest of eggs.
Watanabe, N. et al. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ−TrCP. Proc. Natl Acad. Sci. USA 101, 4419–4424 (2004).
Busino, L. et al. Degradation of Cdc25A by β-TrCP during S phase and in response to DNA damage. Nature 426, 87–91 (2003).
Jin, J. et al. SCFβ–TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 17, 3062–3074 (2003).
Kanemori, Y., Uto, K. & Sagata, N. β-TrCP recognizes a previously undescribed nonphosphorylated destruction motif in Cdc25A and Cdc25B phosphatases. Proc. Natl Acad. Sci. USA 102, 6279–6284 (2005).
Jiang, J. & Struhl, G. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature 391, 493–496 (1998). Shows that a mutation in a D. melanogaster homologue of β-TrCP (Slimb) induces accumulation of a D. melanogaster homologue of β-catenin (Armadillo).
Spevak, W., Keiper, B. D., Stratowa, C. & Castanon, M. J. Saccharomyces cerevisiae cdc15 mutants arrested at a late stage in anaphase are rescued by Xenopus cDNAs encoding N-ras or a protein with β-transducin repeats. Mol. Cell. Biol. 13, 4953–4966 (1993).
Nakayama, K. et al. Impaired degradation of inhibitory subunit of NF-κB (IκB) and β-catenin as a result of targeted disruption of the β-TrCP1 gene. Proc. Natl Acad. Sci. USA 100, 8752–8757 (2003).
Hattori, K., Hatakeyama, S., Shirane, M., Matsumoto, M. & Nakayama, K. I. Molecular dissection of the interactions among IκBα, FWD1, and Skp1 required for ubiquitin-mediated proteolysis of IκBα. J. Biol. Chem. 274, 29641–29647 (1999).
Saitoh, T. & Katoh, M. Expression profiles of βTRCP1 and βTRCP2, and mutation analysis of βTRCP2 in gastric cancer. Int. J. Oncol. 18, 959–964 (2001).
Gerstein, A. V. et al. APC/CTNNB1 (β-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer 34, 9–16 (2002).
Koch, A. et al. Elevated expression of Wnt antagonists is a common event in hepatoblastomas. Clin. Cancer Res. 11, 4295–4304 (2005).
Ougolkov, A. et al. Associations among β-TrCP, an E3 ubiquitin ligase receptor, β-catenin, and NF-κB in colorectal cancer. J. Natl Cancer Inst. 96, 1161–1170 (2004).
Spiegelman, V. S. et al. Wnt/β-catenin signaling induces the expression and activity of βTrCP ubiquitin ligase receptor. Mol. Cell 5, 877–882 (2000).
Muerkoster, S. et al. Increased expression of the E3-ubiquitin ligase receptor subunit βTRCP1 relates to constitutive nuclear factor-κB activation and chemoresistance in pancreatic carcinoma cells. Cancer Res. 65, 1316–1324 (2005).
Kudo, Y. et al. Role of F-box protein βTrcp1 in mammary gland development and tumorigenesis. Mol. Cell. Biol. 24, 8184–8194 (2004).
Belaidouni, N. et al. Overexpression of human βTrCP1 deleted of its F box induces tumorigenesis in transgenic mice. Oncogene 24, 2271–2276 (2005).
Wojcik, E. J., Glover, D. M. & Hays, T. S. The SCF ubiquitin ligase protein slimb regulates centrosome duplication in Drosophila. Curr. Biol. 10, 1131–1134 (2000). Compares the two APC/C degron motifs, the D-box and the KEN-box.
Pfleger, C. M., Lee, E. & Kirschner, M. W. Substrate recognition by the Cdc20 and Cdh1 components of the anaphase-promoting complex. Genes Dev. 15, 2396–2407 (2001).
Pfleger, C. M. & Kirschner, M. W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 14, 655–665 (2000).
Burton JL, Solomon MJ. D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 15, 2381–2395 (2001)
Uhlmann, F., Lottspeich, F. & Nasmyth, K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37–42 (1999).
Uhlmann, F., Wernic, D., Poupart, M. A., Koonin, E. V. & Nasmyth, K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103, 375–386 (2000).
Yanagida, M. Cell cycle mechanisms of sister chromatid separation; roles of Cut1/separin and Cut2/securin. Genes Cells 5, 1–8 (2000).
Bharadwaj, R. & Yu, H. The spindle checkpoint, aneuploidy, and cancer. Oncogene 23, 2016–2027 (2004).
Dominguez, A. et al. hpttg, a human homologue of rat pttg, is overexpressed in hematopoietic neoplasms. Evidence for a transcriptional activation function of hPTTG. Oncogene 17, 2187–2193 (1998).
Hagting, A. et al. Human securin proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J. Cell Biol. 157, 1125–1137 (2002).
Jallepalli, P. V. et al. Securin is required for chromosomal stability in human cells. Cell 105, 445–457 (2001). Shows that human cells without a securin gene lose chromosomes at a high frequency.
Rauh, N. R., Schmidt, A., Bormann, J., Nigg, E. A. & Mayer, T. U. Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature 437, 1048–1052 (2005).
Liu, J. & Maller, J. L. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr. Biol. 15, 1458–1468 (2005).
Wasch, R. & Engelbert, D. Anaphase-promoting complex-dependent proteolysis of cell cycle regulators and genomic instability of cancer cells. Oncogene 24, 1–10 (2005).
Prinz, S., Hwang, E. S., Visintin, R. & Amon, A. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr. Biol. 8, 750–760 (1998).
Zachariae, W., Schwab, M., Nasmyth, K. & Seufert, W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 282, 1721–1724 (1998).
Jaspersen, S. L., Charles, J. F. & Morgan, D. O. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 9, 227–236 (1999).
Visintin, R. et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2, 709–718 (1998).
Bardin, A. J. & Amon, A. Men and sin: what's the difference? Nature Rev. Mol. Cell Biol. 2, 815–826 (2001).
McCollum, D. & Gould, K. L. Timing is everything: regulation of mitotic exit and cytokinesis by the MEN and SIN. Trends Cell Biol. 11, 89–95 (2001).
Simanis, V. The mitotic exit and septation initiation networks. J. Cell Sci. 116, 4261–4262 (2003).
D'Amours, D. & Amon, A. At the interface between signaling and executing anaphase — Cdc14 and the FEAR network. Genes Dev. 18, 2581–2595 (2004).
Wang, C. X., Fisk, B. C., Wadehra, M., Su, H. & Braun, J. Overexpression of murine fizzy-related (fzr) increases natural killer cell-mediated cell death and suppresses tumor growth. Blood 96, 259–263 (2000). The only published study that analyses mutations in subunits of APC/C in human cancers.
Wang, Q. et al. Alterations of anaphase-promoting complex genes in human colon cancer cells. Oncogene 22, 1486–1490 (2003).
Sarafan-Vasseur, N. et al. Overexpression of B-type cyclins alters chromosomal segregation. Oncogene 21, 2051–2057 (2002).
Visintin, R., Prinz, S. & Amon, A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278, 460–463 (1997).
McGarry, T. J. & Kirschner, M. W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93, 1043–1053 (1998).
Melixetian, M. & Helin, K. Geminin: a major DNA replication safeguard in higher eukaryotes. Cell Cycle 3, 1002–1004 (2004).
Carmena, M. & Earnshaw, W. C. The cellular geography of aurora kinases. Nature Rev. Mol. Cell. Biol. 4, 842–854 (2003).
Giet, R., Petretti, C. & Prigent, C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 15, 241–250 (2005).
Zhou, H. et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nature Genet. 20, 189–193 (1998).
Sen, S. et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J. Natl Cancer Inst. 94, 1320–1329 (2002).
Li, D. et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin. Cancer Res. 9, 991–997 (2003).
Ewart-Toland, A. et al. Identification of Stk6/STK15 as a candidate low-penetrance tumor-susceptibility gene in mouse and human. Nature Genet. 34, 403–412 (2003).
Gritsko, T. M. et al. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin. Cancer Res. 9, 1420–1426 (2003).
Hamada, M. et al. Aurora2/BTAK/STK15 is involved in cell cycle checkpoint and cell survival of aggressive non-Hodgkin's lymphoma. Br. J. Haematol. 121, 439–447 (2003).
Meraldi, P., Honda, R. & Nigg, E. A. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 21, 483–492 (2002).
Takai, N., Hamanaka, R., Yoshimatsu, J. & Miyakawa, I. Polo-like kinases (Plks) and cancer. Oncogene 24, 287–291 (2005).
Fry, A. M. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene 21, 6184–6194 (2002).
Hayward, D. G. & Fry, A. M. Nek2 kinase in chromosome instability and cancer. Cancer Lett. 2 Aug 2005 [epub ahead of print].
Vassilev, L. T. et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303, 844–848 (2004).
Ludwig, H., Khayat, D., Giaccone, G. & Facon, T. Proteasome inhibition and its clinical prospects in the treatment of hematologic and solid malignancies. Cancer 104, 1794–1807 (2005).
Cuasck, J. C. Rationale for the treatment of solid tumors with the proteasome inhibitor bortezomib. Cancer Treat. Rev. 29, Suppl. 1 21–31 (2003).
Kudo, Y. et al. High expression of S-phase kinase-interacting protein 2, human F-box protein, correlates with poor prognosis in oral squamous cell carcinomas. Cancer Res. 61, 7044–7047 (2001).
Shintani, S. et al. Skp2 and Jab1 expression are associated with inverse expression of p27KIP1 and poor prognosis in oral squamous cell carcinomas. Oncology 65, 355–362 (2003).
Ben-Izhak, O., Kablan, F., Laster, Z. & Nagler, R. M. Oropharyngeal cancer pathogenesis: ubiquitin proteolytic, apoptotic and epidermal growth factor related pathways act in concert — first report. Oral Oncol. 41, 851–860 (2005).
Dong, Y., Sui, L., Watanabe, Y., Sugimoto, K. & Tokuda, M. S-phase kinase-associated protein 2 expression in laryngeal squamous cell carcinomas and its prognostic implications. Oncol. Rep. 10, 321–325 (2003).
Gstaiger, M. et al. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl Acad. Sci. USA 98, 5043–5048 (2001).
Fukuchi, M. et al. Inverse correlation between expression levels of p27 and the ubiquitin ligase subunit Skp2 in early esophageal squamous cell carcinoma. Anticancer Res. 24, 777–783 (2004).
Masuda, T. A. et al. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 62, 3819–3825 (2002).
Honjo, S. et al. COX-2 correlates with F-box protein, Skp2 expression and prognosis in human gastric carcinoma. Int. J. Oncol. 26, 353–360 (2005).
Hershko, D. et al. Inverse relation between levels of p27Kip1 and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer 91, 1745–1751 (2001).
Li, J. Q. et al. Correlation of Skp2 with carcinogenesis, invasion, metastasis, and prognosis in colorectal tumors. Int. J. Oncol. 25, 87–95 (2004).
Sanada, T. et al. Skp2 overexpression is a p27Kip1-independent predictor of poor prognosis in patients with biliary tract cancers. Cancer Sci. 95, 969–976 (2004).
Zhu, C. Q. et al. Skp2 gene copy number aberrations are common in non-small cell lung carcinoma, and its overexpression in tumors with ras mutation is a poor prognostic marker. Clin. Cancer Res. 10, 1984–1991 (2004).
Goto, A. et al. Immunohistochemical study of Skp2 and Jab1, two key molecules in the degradation of P27, in lung adenocarcinoma. Pathol. Int. 54, 675–681 (2004).
Osoegawa, A. et al. Regulation of p27 by S-phase kinase-associated protein 2 is associated with aggressiveness in non-small-cell lung cancer. J. Clin. Oncol. 22, 4165–4173 (2004).
Takanami, I. The prognostic value of overexpression of Skp2 mRNA in non-small cell lung cancer. Oncol. Rep. 13, 727–731 (2005).
Li, Q., Murphy, M., Ross, J., Sheehan, C. & Carlson, J. A. Skp2 and p27kip1 expression in melanocytic nevi and melanoma: an inverse relationship. J. Cutan. Pathol. 31, 633–642 (2004).
Woenckhaus, C. et al. Expression of Skp2 and p27KIP1 in naevi and malignant melanoma of the skin and its relation to clinical outcome. Histol. Histopathol. 20, 501–508 (2005).
Schiffer, D., Cavalla, P., Fiano, V., Ghimenti, C. & Piva, R. Inverse relationship between p27/Kip.1 and the F-box protein Skp2 in human astrocytic gliomas by immunohistochemistry and Western blot. Neurosci. Lett. 328, 125–128 (2002).
Saigusa, K. et al. Overexpressed Skp2 within 5p amplification detected by array-based comparative genomic hybridization is associated with poor prognosis of glioblastomas. Cancer Sci. 96, 676–683 (2005).
Langner, C., von Wasielewski, R., Ratschek, M., Rehak, P. & Zigeuner, R. Biological significance of p27 and Skp2 expression in renal cell carcinoma. A systematic analysis of primary and metastatic tumour tissues using a tissue microarray technique. Virchows Arch. 445, 631–636 (2004).
Yang, G. et al. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin. Cancer Res. 8, 3419–3426 (2002).
Ben-Izhak, O. et al. Inverse relationship between Skp2 ubiquitin ligase and the cyclin dependent kinase inhibitor p27Kip1 in prostate cancer. J. Urol. 170, 241–245 (2003).
Drobnjak, M. et al. Altered expression of p27 and Skp2 proteins in prostate cancer of African-American patients. Clin. Cancer Res. 9, 2613–2619 (2003).
Langner, C., von Wasielewski, R., Ratschek, M., Rehak, P. & Zigeuner, R. Expression of p27 and its ubiquitin ligase subunit Skp2 in upper urinary tract transitional cell carcinoma. Urology 64, 611–616 (2004).
Dowen, S. E., Scott, A., Mukherjee, G. & Stanley, M. A. Overexpression of Skp2 in carcinoma of the cervix does not correlate inversely with p27 expression. Int. J. Cancer 105, 326–330 (2003).
Lahav-Baratz, S. et al. Decreased level of the cell cycle regulator p27 and increased level of its ubiquitin ligase Skp2 in endometrial carcinoma but not in normal secretory or in hyperstimulated endometrium. Mol. Hum. Reprod. 10, 567–572 (2004).
Shigemasa, K., Gu, L., O'Brien, T. J. & Ohama, K. Skp2 overexpression is a prognostic factor in patients with ovarian adenocarcinoma. Clin. Cancer Res. 9, 1756–1763 (2003).
Penin, R. M. et al. Over-expression of p45(SKP2) in Kaposi's sarcoma correlates with higher tumor stage and extracutaneous involvement but is not directly related to p27KIP1 down-regulation. Mod. Pathol. 15, 1227–1235 (2002).
Oliveira, A. M., Okuno, S. H., Nascimento, A. G. & Lloyd, R. V. Skp2 protein expression in soft tissue sarcomas. J. Clin. Oncol. 21, 722–727 (2003).
Chiarle, R. et al. S-phase kinase-associated protein 2 expression in non-Hodgkin's lymphoma inversely correlates with p27 expression and defines cells in S phase. Am. J. Pathol. 160, 1457–1466 (2002).
Seki, R. et al. Prognostic significance of the F-box protein Skp2 expression in diffuse large B-cell lymphoma. Am. J. Hematol. 73, 230–235 (2003).
Min, Y. H. et al. Elevated S-phase kinase-associated protein 2 protein expression in acute myelogenous leukemia: its association with constitutive phosphorylation of phosphatase and tensin homologue protein and poor prognosis. Clin. Cancer Res. 10, 5123–5130 (2004).
Lim, M. S. et al. Expression of Skp2, a p27Kip1 ubiquitin ligase, in malignant lymphoma: correlation with p27Kip1 and proliferation index. Blood 100, 2950–2956 (2002).
Kwak, E. L. et al. Infrequent mutations of Archipelago (hAGO, hCDC4, Fbw7) in primary ovarian cancer. Gynecol. Oncol. 98, 124–128 (2005).
Strohmaier, H. et al. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413, 316–322 (2001).
Spruck, C. H. et al. hCDC4 gene mutations in endometrial cancer. Cancer Res. 62, 4535–4539 (2002).
Cassia, R. et al. Cyclin E gene (CCNE) amplification and hCDC4 mutations in endometrial carcinoma. J. Pathol. 201, 589–595 (2003).
Li, D. et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin. Cancer Res. 9, 991–997 (2003).
Singhal, S. et al. Alterations in cell cycle genes in early stage lung adenocarcinoma identified by expression profiling. Cancer Biol. Ther. 2, 291–298 (2003).
Kim, J. M. et al. Identification of gastric cancer-related genes using a cDNA microarray containing novel expressed sequence tags expressed in gastric cancer cells. Clin. Cancer Res. 11, 473–482 (2005).
Acknowledgements
The authors wish to thank all members of our laboratories for their comments. We are also thankful to A. Ohta and M. Kimura for help in preparing this manuscript. This research was partly supported by a grant from CREST, Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary table S1, supplementary references and figures S2-S3 (PDF 405 kb)
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Glossary
- Cyclin-dependent kinase
-
A protein kinase that controls cell-cycle progression in all eukaryotes and requires physical association with cyclins to achieve full enzymatic activity.
- Cyclin
-
The positive regulatory subunits of cyclin-dependent kinases, showing oscillating expression in the cell cycle.
- Ubiquitin
-
A 76-amino-acid polypeptide that is conjugated through an isopeptide linkage to other proteins. Such conjugates are most commonly targeted for degradation by the proteasome.
- Proteasome
-
A large ∼2.5-MDa multisubunit protein complex that binds to and subsequently degrades polyubiquitylated proteins in an ATP-dependent manner.
- Ubiquitin-conjugating enzyme (E2)
-
An enzyme that accepts ubiquitin from a ubiquitin-activating enzyme (E1) and, together with a ubiquitin ligase (E3), transfers it to a substrate protein.
- Ubiquitin ligase (E3)
-
A protein or protein complex that facilitates the transfer of ubiquitin from a ubiquitin-conjugating enzyme (E2) to a substrate. E3 enzymes provide platforms for binding E2 enzymes and specific substrates, thereby coordinating the ubiquitylation of the selected substrate.
- Cullins
-
A family of proteins that are characterized by the presence of a distinct globular C-terminal domain (cullin homology domain) and a series of N-terminal repeats of a five helix bundle (cullin repeats).
- F-box protein
-
A variable component of SCF E3 ligase that binds to SKP1 through the Fbox domain. FBPs recognize specific substrates and, with the help of other subunits of the E3 ubiquitin ligase, deliver them to the E2 ubiquitin-conjugating enzyme.
- RING-finger proteins
-
A family of proteins structurally defined by a particular folded protein domain that binds Zn2+ through a four-point arrangement of cysteine and histidine amino acids. In most cases, a RING-finger protein interacts with an E2 and serves as an E3.
- WD40 repeat
-
A protein-interaction domain consisting of 40 amino-acid repeats that form a propeller-like structure, in which each repeat contributes a blade.
- Leucine-rich repeat
-
A protein-sequence motif that contains regular occurrences of the amino acid leucine, which are present as tandem arrays in certain proteins. The back-to-back set of motifs was found to correspond to a small sub-domain structure in the protein that stacks next to adjacent repeats to form a parallel, β-sheet, arc-like structure.
- Degron
-
A portion of a protein that is necessary and sufficient to bring about its degradation by the ubiquitin–proteasome system.
- Spindle
-
A highly dynamic, bipolar array of microtubules that forms during mitosis or meiosis and serves to move the duplicated chromosomes apart.
- Kinetochore
-
The complicated protein assembly that links the specialized areas of condensed chromosomes, known as centromeres, to the microtubule-based mitotic spindle.
- Aneuploidy
-
The ploidy of a cell refers to the number of sets of chromosomes that it contains. Aneuploid karyotypes are those of which chromosome complements are not a simple multiple of the haploid set.
Rights and permissions
About this article
Cite this article
Nakayama, K., Nakayama, K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer 6, 369–381 (2006). https://doi.org/10.1038/nrc1881
Issue Date:
DOI: https://doi.org/10.1038/nrc1881
This article is cited by
-
Deciphering the role of neddylation in tumor microenvironment modulation: common outcome of multiple signaling pathways
Biomarker Research (2024)
-
FBXW7 regulates the sensitivity of imatinib in gastrointestinal stromal tumors by targeting MCL1
Gastric Cancer (2024)
-
PRPF19 facilitates colorectal cancer liver metastasis through activation of the Src-YAP1 pathway via K63-linked ubiquitination of MYL9
Cell Death & Disease (2023)
-
Deubiquitinases in cancer
Nature Reviews Cancer (2023)
-
FBXW2 suppresses breast tumorigenesis by targeting AKT-Moesin-SKP2 axis
Cell Death & Disease (2023)