Abstract
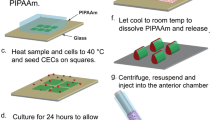
The mouse corneal micropocket angiogenesis assay uses the avascular cornea as a canvas to study angiogenesis in vivo. Through the use of standardized slow-release pellets, a predictable angiogenic response is generated over the course of 5 d and then quantified. Uniform slow-release pellets are prepared by mixing purified angiogenic growth factors such as basic fibroblast growth factor or vascular endothelial growth factor with sucralfate (a stabilizer) and Hydron (poly-HEMA (poly(2-hydroxyethyl methacrylate)) to allow slow release). This mixture is applied to a mesh that controls unit size and then allowed to harden. A micropocket is surgically created in the mouse cornea and a pellet implanted. Five days later, the area of the cornea overgrown by the angiogenic response is measured using a slit lamp. A skilled investigator can implant and grade 40 eyes in about 2.5 h. The results of the assay are used to assess the ability of potential therapeutic molecules or genetic differences to modulate angiogenesis in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Gospodarowicz, D., Moran, J., Braun, D. & Birdwell, C. Clonal growth of bovine vascular endothelial cells: fibroblast growth factor as a survival agent. Proc. Natl. Acad. Sci. USA 73, 4120–4124 (1976).
Glaser, B.M., D'Amore, P.A., Seppa, H., Seppa, S. & Schiffmann, E. Adult tissues contain chemoattractants for vascular endothelial cells. Nature 288, 483–484 (1980).
Kubota, Y., Kleinman, H.K., Martin, G.R. & Lawley, T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 107, 1589–1598 (1988).
Montesano, R. & Orci, L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell 42, 469–477 (1985).
Korff, T. & Augustin, H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell Biol. 143, 1341–1352 (1998).
Nicosia, R.F., Tchao, R. & Leighton, J. Histotypic angiogenesis in vitro: light microscopic, ultrastructural, and radioautographic studies. In Vitro 18, 538–549 (1982).
Sandison, J.C. A new method for the microscopic study of living growing tissues by the introduction of a transparent chamber in the rabbit's ear. Anat. Rec. 28, 281–287 (1924).
Jain, R.K., Schlenger, K., Hockel, M. & Yuan, F. Quantitative angiogenesis assays: progress and problems. Nat. Med. 3, 1203–1208 (1997).
Gimbrone, M.A., Leapman, S.B., Cotran, R.S. & Folkman, J. Tumor dormancy in vivo by prevention of neovascularization. J. Exp. Med. 136, 261–276 (1972).
Maiorana, A. & Gullino, P.M. Acquisition of angiogenic capacity and neoplastic transformation in the rat mammary gland. Cancer Res. 38, 4409–4414 (1978).
Gimbrone, M.A., Cotran, R.S., Leapman, S.B. & Folkman, J. Tumor growth and neovascularization: an experimental model using the rabbit cornea. J. Natl. Cancer Inst. 52, 413–427 (1974).
Gimbrone, M.A., Leapman, S.B., Cotran, R.S. & Folkman, J. Tumor angiogenesis: iris neovascularization at a distance from experimental intraocular tumors. J. Natl. Cancer Inst. 50, 219–228 (1973).
Muthukkaruppan, V. & Auerbach, R. Angiogenesis in the mouse cornea. Science 205, 1416–1418 (1979).
D'Amato, R.J., Loughnan, M.S., Flynn, E. & Folkman, J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA 91, 4082–4085 (1994).
Li, W.W., Grayson, G., Folkman, J. & D'Amore, P.A. Sustained-release endotoxin. A model for inducing corneal neovascularization. Invest. Ophthalmol. Vis. Sci. 32, 2906–2911 (1991).
Phillips, G.D. et al. Vascular endothelial growth factor (rhVEGF165) stimulates direct angiogenesis in the rabbit cornea. In Vivo 8, 961–965 (1994).
BenEzra, D., Hemo, I. & Maftzir, G. In vivo angiogenic activity of interleukins. Arch. Ophthalmol. 108, 573–576 (1990).
Knighton, D.R., Phillips, G.D. & Fiegel, V.D. Wound healing angiogenesis: indirect stimulation by basic fibroblast growth factor. J. Trauma 30, S134–S144 (1990).
Kenyon, B.M. et al. A model of angiogenesis in the mouse cornea. Invest. Ophthalmol. Vis. Sci. 37, 1625–1632 (1996).
Chang, L.K. et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc. Natl. Acad. Sci. USA 101, 11658–11663 (2004).
Hoang, M.V., Whelan, M.C. & Senger, D.R. Rho activity critically and selectively regulates endothelial cell organization during angiogenesis. Proc. Natl. Acad. Sci. USA 101, 1874–1879 (2004).
Rogers, M.S., Rohan, R.M., Birsner, A.E. & D'Amato, R.J. Genetic loci that control vascular endothelial growth factor-induced angiogenesis. FASEB J. 17, 2112–2114 (2003).
Rogers, M.S. & D'Amato, R.J. The effect of genetic diversity on angiogenesis. Exp. Cell Res. 312, 561–574 (2006).
Rogers, M.S., Rohan, R.M., Birsner, A.E. & D'Amato, R.J. Genetic loci that control the angiogenic response to basic fibroblast growth factor. FASEB J. 18, 1050–1059 (2004).
Rohan, R.M., Fernandez, A., Udagawa, T., Yuan, J. & D'Amato, R.J. Genetic heterogeneity of angiogenesis in mice. FASEB J. 14, 871–876 (2000).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Video 1
Video showing the formation of a corneal micropocket and pellet implantation (MOV 5822 kb)
Rights and permissions
About this article
Cite this article
Rogers, M., Birsner, A. & D'Amato, R. The mouse cornea micropocket angiogenesis assay. Nat Protoc 2, 2545–2550 (2007). https://doi.org/10.1038/nprot.2007.368
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.368
This article is cited by
-
Akt1-dependent expression of angiopoietin 1 and 2 in vascular smooth muscle cells leads to vascular stabilization
Experimental & Molecular Medicine (2022)
-
Capillary morphogenesis gene 2 (CMG2) mediates growth factor-induced angiogenesis by regulating endothelial cell chemotaxis
Angiogenesis (2022)
-
Targeting HIF-1α by newly synthesized Indolephenoxyacetamide (IPA) analogs to induce anti-angiogenesis-mediated solid tumor suppression
Pharmacological Reports (2021)
-
Low dose amiodarone reduces tumor growth and angiogenesis
Scientific Reports (2020)
-
Modelling of endothelial cell migration and angiogenesis in microfluidic cell culture systems
Biomechanics and Modeling in Mechanobiology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.