Abstract
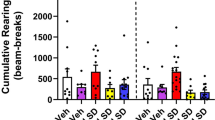
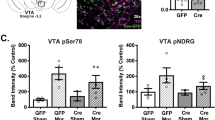
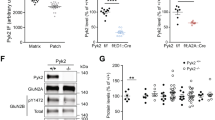
The widespread distribution of the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10) in the adult brain1 suggests its role in a broad range of brain functions. Here we show evidence supporting a physical interaction of PTEN with a region in the third intracellular loop (3L4F) of the serotonin 5-HT2C receptor (5-HT2cR, formerly 5-HT1c receptor2) in cell cultures. PTEN limits agonist-induced phosphorylation of 5-HT2cR through its protein phosphatase activity. We showed the probable existence of PTEN:5-HT2cR complexes in putative dopaminergic neurons in the rat ventral tegmental area (VTA), a brain region in which virtually all abused drugs exert rewarding effects by activating its dopamine neurons3,4. We synthesized the interfering peptide Tat-3L4F, which is able to disrupt PTEN coupling with 5-HT2cR. Systemic application of Tat-3L4F or the 5-HT2cR agonist Ro600175 suppressed the increased firing rate of VTA dopaminergic neurons induced by Δ9-tetrahydrocannabinol (THC), the psychoactive ingredient of marijuana. Using behavioral tests, we found that Tat-3L4F or Ro600175 blocks conditioned place preference of THC or nicotine, and that Ro600175, but not Tat-3L4F, produces anxiogenic effects, penile erection, hypophagia and motor functional suppression. These results suggest a potential strategy for treating drug addiction with the Tat-3L4F peptide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lachyankar, M.B. et al. A role for nuclear PTEN in neuronal differentiation. J. Neurosci. 20, 1404–1413 (2000).
Julius, D., MacDermott, A.B., Axel, R. & Jessell, T.M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science 241, 558–564 (1988).
Lupica, C.R., Riegel, A.C. & Hoffman, A.F. Marijuana and cannabinoid regulation of brain reward circuits. Br. J. Pharmacol. 143, 227–234 (2004).
Nestler, E.J. Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol. Sci. 25, 210–218 (2004).
Steelman, L.S., Bertrand, F.E. & McCubrey, J.A. The complexity of PTEN: mutation, marker and potential target for therapeutic intervention. Expert Opin. Ther. Targets 8, 537–550 (2004).
Li, L., Liu, F. & Ross, A.H. PTEN regulation of neural development and CNS stem cells. J. Cell. Biochem. 88, 24–28 (2003).
Ning, K. et al. Dual neuroprotective signaling mediated by downregulating two distinct phosphatase activities of PTEN. J. Neurosci. 24, 4052–4060 (2004).
Backstrom, J.R., Price, R.D., Reasoner, D.T. & Sanders-Bush, E. Deletion of the serotonin 5–HT2C receptor PDZ recognition motif prevents receptor phosphorylation and delays resensitization of receptor responses. J. Biol. Chem. 275, 23620–23626 (2000).
Tamura, M. et al. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science 280, 1614–1617 (1998).
Schwarze, S.R., Ho, A., Vocero-Akbani, A. & Dowdy, S.F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572 (1999).
Furnari, F.B., Lin, H., Huang, H.S. & Cavenee, W.K. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc. Natl. Acad. Sci. USA 94, 12479–12484 (1997).
Weng, L.P., Brown, J.L. & Eng, C. PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum. Mol. Genet. 10, 599–604 (2001).
Pompeiano, M., Palacios, J.M. & Mengod, G. Distribution of the serotonin 5–HT2 receptor family mRNAs: comparison between 5–HT2A and 5–HT2C receptors. Brain Res. Mol. Brain Res. 23, 163–178 (1994).
Di Matteo, V., Cacchio, M., Di Giulio, C. & Esposito, E. Role of serotonin(2C) receptors in the control of brain dopaminergic function. Pharmacol. Biochem. Behav. 71, 727–734 (2002).
Higgins, G.A. & Fletcher, P.J. Serotonin and drug reward: focus on 5–HT2C receptors. Eur. J. Pharmacol. 480, 151–162 (2003).
Aarts, M. et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 298, 846–850 (2002).
Clifton, P.G., Lee, M.D. & Dourish, C.T. Similarities in the action of Ro 60–0175, a 5–HT2C receptor agonist, and d-fenfluramine on feeding patterns in the rat. Psychopharmacology (Berl.) 152, 256–267 (2000).
Grottick, A.J., Fletcher, P.J. & Higgins, G.A. Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J. Pharmacol. Exp. Ther. 295, 1183–1191 (2000).
Alves, S.H., Pinheiro, G., Motta, V., Landeira-Fernandez, J. & Cruz, A.P. Anxiogenic effects in the rat elevated plus-maze of 5-HT(2C) agonists into ventral but not dorsal hippocampus. Behav. Pharmacol. 15, 37–43 (2004).
Wood, M.D. Therapeutic potential of 5–HT2C receptor antagonists in the treatment of anxiety disorders. Curr. Drug Targets CNS Neurol. Disord. 2, 383–387 (2003).
Cheer, J.F., Marsden, C.A., Kendall, D.A. & Mason, R. Lack of response suppression follows repeated ventral tegmental cannabinoid administration: an in vitro electrophysiological study. Neuroscience. 99, 661–667 (2000).
Johnson, S.W. & North, R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 12, 483–488 (1992).
Szabo, B., Siemes, S. & Wallmichrath, I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur. J. Neurosci. 15, 2057–2061 (2002).
Backstrom, J.R. & Sanders-Bush, E. Generation of anti-peptide antibodies against serotonin 5–HT2A and 5–HT2C receptors. J. Neurosci. Methods 77, 109–117 (1997).
Zhang, X. et al. Susceptibility to kindling and neuronal connections of the anterior claustrum. J. Neurosci. 21, 3674–3687 (2001).
Paxinos, G. & Watson, C. The rat brain stereotaxic co-ordinates, 2nd edition. (Academic Press, New York, 1986).
Bunney, B.S., Walters, J.R., Roth, R.H. & Aghajanian, G.K. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J. Pharmacol. Exp. Ther. 185, 560–571 (1973).
Braida, D., Iosue, S., Pegorini, S. & Sala, M. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur. J. Pharmacol. 506, 63–69 (2004).
Hannesson, D.K. et al. Dorsal hippocampal kindling produces a selective and enduring disruption of hippocampally mediated behavior. J. Neurosci. 21, 4443–4450 (2001).
Rice, H.B. & Corwin, R.L. mCPP-induced hypophagia in rats is unaffected by the profile of dietary unsaturated fatty acids. Pharmacol. Biochem. Behav. 73, 545–550 (2002).
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and Natural Sciences and Engineering Research Council of Canada awarded to X.Z., who is the recipient of the CIHR New Investigator Award. S.-P.J. and W.J. were supported by Postdoctoral Fellowship Awards from the Saskatchewan Health Research Foundation, Canada. We thank Y. Li and G. Kort for technical assistance and X. Wu for confocal imaging. G129R and G129E were provided by F. Furnari at the Ludwig Institute for Cancer Research, University of California at San Diego. We also thank M. Tamura at the US National Institutes of Health for providing wild-type PTEN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ji, SP., Zhang, Y., Van Cleemput, J. et al. Disruption of PTEN coupling with 5-HT2C receptors suppresses behavioral responses induced by drugs of abuse. Nat Med 12, 324–329 (2006). https://doi.org/10.1038/nm1349
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1349