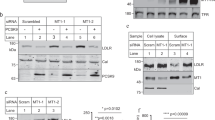
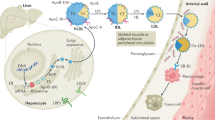
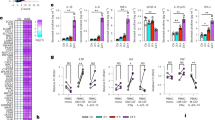
Abstract
Recent studies have indicated that high-density lipoproteins (HDLs) and their major structural protein, apolipoprotein A1 (apoA1), recovered from human atheroma are dysfunctional and are extensively oxidized by myeloperoxidase (MPO). In vitro oxidation of either apoA1 or HDL particles by MPO impairs their cholesterol acceptor function. Here, using phage display affinity maturation, we developed a high-affinity monoclonal antibody that specifically recognizes both apoA1 and HDL that have been modified by the MPO-H2O2-Cl− system. An oxindolyl alanine (2-OH-Trp) moiety at Trp72 of apoA1 is the immunogenic epitope. Mutagenesis studies confirmed a critical role for apoA1 Trp72 in MPO-mediated inhibition of the ATP-binding cassette transporter A1 (ABCA1)-dependent cholesterol acceptor activity of apoA1 in vitro and in vivo. ApoA1 containing a 2-OH-Trp72 group (oxTrp72-apoA1) is in low abundance within the circulation but accounts for 20% of the apoA1 in atherosclerosis-laden arteries. OxTrp72-apoA1 recovered from human atheroma or plasma is lipid poor, virtually devoid of cholesterol acceptor activity and demonstrated both a potent proinflammatory activity on endothelial cells and an impaired HDL biogenesis activity in vivo. Elevated oxTrp72-apoA1 levels in subjects presenting to a cardiology clinic (n = 627) were associated with increased cardiovascular disease risk. Circulating oxTrp72-apoA1 levels may serve as a way to monitor a proatherogenic process in the artery wall.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Barter, P.J. et al. Antiinflammatory properties of HDL. Circ. Res. 95, 764–772 (2004).
Duffy, D. & Rader, D.J. Update on strategies to increase HDL quantity and function. Nat. Rev. Cardiol. 6, 455–463 (2009).
Navab, M., Reddy, S.T., Van Lenten, B.J. & Fogelman, A.M. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 8, 222–232 (2011).
Khera, A.V. et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364, 127–135 (2011).
Vickers, K.C., Palmisano, B.T., Shoucri, B.M., Shamburek, R.D. & Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 13, 423–433 (2011).
Fisher, E.A., Feig, J.E., Hewing, B., Hazen, S.L. & Smith, J.D. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 32, 2813–2820 (2012).
Gordon, T., Castelli, W.P., Hjortland, M.C., Kannel, W.B. & Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62, 707–714 (1977).
Badimon, J.J., Badimon, L., Galvez, A., Dische, R. & Fuster, V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab. Invest. 60, 455–461 (1989).
Badimon, J.J., Badimon, L. & Fuster, V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 85, 1234–1241 (1990).
Rubin, E.M., Krauss, R.M., Spangler, E.A., Verstuyft, J.G. & Clift, S.M. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature 353, 265–267 (1991).
Plump, A.S., Scott, C.J. & Breslow, J.L. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E–deficient mouse. Proc. Natl. Acad. Sci. USA 91, 9607–9611 (1994).
Hughes, S.D., Verstuyft, J. & Rubin, E.M. HDL deficiency in genetically engineered mice requires elevated LDL to accelerate atherogenesis. Arterioscler. Thromb. Vasc. Biol. 17, 1725–1729 (1997).
Nissen, S.E. et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. J. Am. Med. Assoc. 290, 2292–2300 (2003).
Sacks, F.M. et al. Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J. Lipid Res. 50, 894–907 (2009).
Tardif, J.C. et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. J. Am. Med. Assoc. 297, 1675–1682 (2007).
Barter, P.J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Nissen, S.E. et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N. Engl. J. Med. 356, 1304–1316 (2007).
Boden, W.E. et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 (2011).
Voight, B.F. et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 380, 572–580 (2012).
Bhattacharyya, T. et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. J. Am. Med. Assoc. 299, 1265–1276 (2008).
Besler, C. et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest. 121, 2693–2708 (2011).
Sorci-Thomas, M.G. & Thomas, M.J. High density lipoprotein biogenesis, cholesterol efflux, and immune cell function. Arterioscler. Thromb. Vasc. Biol. 32, 2561–2565 (2012).
Shih, D.M. et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J. Biol. Chem. 275, 17527–17535 (2000).
Tang, W.H. et al. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 32, 2803–2812 (2012).
DiDonato, J.A. et al. Function and distribution of apolipoprotein A1 in the artery wall are markedly distinct from those in plasma. Circulation 128, 1644–1655 (2013).
Zheng, L. et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114, 529–541 (2004).
Wu, Z. et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat. Struct. Mol. Biol. 14, 861–868 (2007).
Peng, D.Q. et al. Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler. Thromb. Vasc. Biol. 28, 2063–2070 (2008).
Undurti, A. et al. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J. Biol. Chem. 284, 30825–30835 (2009).
Hadfield, K.A. et al. Myeloperoxidase-derived oxidants modify apolipoprotein A-I and generate dysfunctional high-density lipoproteins: comparison of hypothiocyanous acid (HOSCN) with hypochlorous acid (HOCl). Biochem. J. 449, 531–542 (2013).
Van Lenten, B.J. et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Invest. 96, 2758–2767 (1995).
Ansell, B.J. et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108, 2751–2756 (2003).
Charles-Schoeman, C. et al. Effects of high-dose atorvastatin on antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. J. Rheumatol. 34, 1459–1464 (2007).
Shao, B., Pennathur, S. & Heinecke, J.W. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J. Biol. Chem. 287, 6375–6386 (2012).
Brennan, M.L. et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 277, 17415–17427 (2002).
Timmins, J.M. et al. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115, 1333–1342 (2005).
Barter, P.J. & Kastelein, J.J. Targeting cholesteryl ester transfer protein for the prevention and management of cardiovascular disease. J. Am. Coll. Cardiol. 47, 492–499 (2006).
Bergt, C. et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA 101, 13032–13037 (2004).
Shao, B. et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1-dependent cholesterol transport. J. Biol. Chem. 280, 5983–5993 (2005).
Cybulsky, M.I. et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. 107, 1255–1262 (2001).
Segrest, J.P. et al. A detailed molecular belt model for apolipoprotein A-I in discoidal high density lipoprotein. J. Biol. Chem. 274, 31755–31758 (1999).
Wu, Z. et al. Double superhelix model of high density lipoprotein. J. Biol. Chem. 284, 36605–36619 (2009).
Gogonea, V. et al. Congruency between biophysical data from multiple platforms and molecular dynamics simulation of the double-super helix model of nascent high-density lipoprotein. Biochemistry 49, 7323–7343 (2010).
Wu, Z. et al. The low resolution structure of ApoA1 in spherical high density lipoprotein revealed by small angle neutron scattering. J. Biol. Chem. 286, 12495–12508 (2011).
Gogonea, V. et al. The low-resolution structure of nHDL reconstituted with DMPC with and without cholesterol reveals a mechanism for particle expansion. J. Lipid Res. 54, 966–983 (2013).
Pattison, D.I. & Davies, M.J. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 14, 1453–1464 (2001).
Baldus, S. et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J. Clin. Invest. 108, 1759–1770 (2001).
Abu-Soud, H.M. & Hazen, S.L. Nitric oxide is a physiological substrate for mammalian peroxidases. J. Biol. Chem. 275, 37524–37532 (2000).
Eiserich, J.P. et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 296, 2391–2394 (2002).
Wang, Y. et al. Myeloperoxidase inactivates TIMP-1 by oxidizing its N-terminal cysteine residue: an oxidative mechanism for regulating proteolysis during inflammation. J. Biol. Chem. 282, 31826–31834 (2007).
Sugiyama, S. et al. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler. Thromb. Vasc. Biol. 24, 1309–1314 (2004).
Nahrendorf, M. et al. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation 117, 1153–1160 (2008).
Ronald, J.A. et al. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation 120, 592–599 (2009).
Hazen, S.L. & Heinecke, J.W. 3-chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J. Clin. Invest. 99, 2075–2081 (1997).
Zamanian-Daryoush, M. et al. The cardioprotective protein apolipoprotein A1 promotes potent anti-tumorigenic effects. J. Biol. Chem. 288, 21237–21252 (2013).
Markwell, M.A., Haas, S.M., Bieber, L.L. & Tolbert, N.E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87, 206–210 (1978).
Ryan, R.O., Forte, T.M. & Oda, M.N. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr. Purif. 27, 98–103 (2003).
Matz, C.E. & Jonas, A. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J. Biol. Chem. 257, 4535–4540 (1982).
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Todorovski, T., Fedorova, M., Hennig, L. & Hoffmann, R. Synthesis of peptides containing 5-hydroxytryptophan, oxindolylalanine, N-formylkynurenine and kynurenine. J. Pept. Sci. 17, 256–262 (2011).
Ståhlman, M. et al. Proteomics and lipids of lipoproteins isolated at low salt concentrations in D2O/sucrose or in KBr. J. Lipid Res. 49, 481–490 (2008).
Robinet, P., Wang, Z., Hazen, S.L. & Smith, J.D. A simple and sensitive enzymatic method for cholesterol quantification in macrophages and foam cells. J. Lipid Res. 51, 3364–3369 (2010).
Barbas, C.F. III, Burton, D.R., Scott, J.K. & Silverman, G.J. Phage Display: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2001).
Marks, J.D. et al. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222, 581–597 (1991).
Chung, S. et al. Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism. J. Biol. Chem. 285, 12197–12209 (2010).
Acknowledgements
We thank M. Liang (Chinese Center for Disease Control and Prevention) for the gift of the dicistronic baculoviral shuttle vector used to subclone the scFv gene. This study was supported by US National Institutes of Health (NIH) grants P01HL098055 and HL119962. BioBank, the clinical study from which samples were analyzed, was supported in part from NIH grants P01HL098055, P01HL076491, R01HL103866, P20HL113452 and R01HL103931. This work was also supported in part by a grant from the LeDucq Fondation. S.L.H. is also partially supported by a gift from the Leonard Krieger Fund. Mass spectrometry instrumentation used was housed within the Cleveland Clinic Mass Spectrometry Facility, which is partially supported through a Center of Innovation Award by AB SCIEX.
Author information
Authors and Affiliations
Contributions
Y.H. participated in all laboratory, animal and human studies, assisted in statistical analyses, helped design the experiments and drafted the manuscript. B.S.L., G.S.G., V.G., C.S.K., Z.W. and X.F. assisted with various laboratory and mass spectrometry studies. D.S., J.B., M.K.C., S.Z.B. and C.-C.C. helped perform various animal experiments. J.A.D., D.S., T.K., X.G., M.K.C., J.E.H., A.J.D. and D.P. helped make various bacterial expression clones and produce and purify recombinant proteins used. J.A.D. and S.L. helped with mAb generation and screening. T.K. and T.T.N. helped with ELISA assays. L.L. and Y.W. provided statistical analyses of clinical data. J.A.D., L.C., E.F.P., P.L.F., V.G., W.H.W.T., J.S.P., E.A.F., J.D.S. and S.L.H. provided experimental analysis and expertise. All authors took part in critical review of the manuscript. The project was scientifically conceived and directed by S.L.H.
Corresponding author
Ethics declarations
Competing interests
W.H.W.T. has previously received research grant support from Abbott Laboratories. S.L.H., Z.W., B.S.L. and J.D.S. report being listed as co-inventors on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics or therapeutics. S.L.H. reports having been paid as a consultant for the following companies: AstraZeneca Pharmaceuticals LP, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., Pfizer Inc., Procter & Gamble and Takeda. S.L.H. reports receiving research funds from Cleveland Heart Lab, Liposcience Inc., Procter & Gamble and Takeda. J.D.S. reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab and Esperion and being paid as a consultant for Esperion. S.L.H. reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the following companies: Cleveland Heart Lab, Esperion, Frantz Biomarkers, LLC and Liposcience Inc. B.S.L. and Z.W. report having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics from Liposcience Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–2, Supplementary Figures 1–8 (PDF 2429 kb)
Rights and permissions
About this article
Cite this article
Huang, Y., DiDonato, J., Levison, B. et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat Med 20, 193–203 (2014). https://doi.org/10.1038/nm.3459
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3459
This article is cited by
-
Optimization of ultrasound-assisted extraction of phenolic compounds from Populus nigra as potential myeloperoxidase inhibitors
Chemical Papers (2024)
-
Association between serum apolipoprotein A1 and atrial fibrillation in the Chinese population: a case–control study
BMC Cardiovascular Disorders (2023)
-
Fully automated immunoassay for cholesterol uptake capacity to assess high-density lipoprotein function and cardiovascular disease risk
Scientific Reports (2023)
-
Effects of superoxide anion attack on the lipoprotein HDL
Molecular and Cellular Biochemistry (2023)
-
The association between impairment of HDL cholesterol efflux capacity and atrial remodeling in atrial fibrillation
Scientific Reports (2021)