Abstract
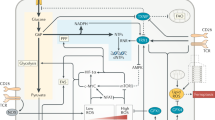
Oxidants such as H2O2 are connected to lymphocyte activation, but the molecular mechanisms behind this phenomenon are less clear. Here, I review data suggesting that by inhibiting protein tyrosine phosphatases, H2O2 plays an important role as a secondary messenger in the initiation and amplification of signaling at the antigen receptor. These findings explain why exposure of lymphocytes to H2O2 can mimic the effect of antigen. In addition, more recent data show that antigen receptors themselves are H2O2-generating enzymes and that the oxidative burst in macrophages seems to play a role not only in pathogen killing but also in the activation of these as well as neighboring cells. Thus, by controlling the activity of the negative regulatory phosphatases inside the cell, H2O2 can set and influence critical thresholds for lymphocyte activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Finkel, T. & Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 (2000).
Adler, V., Yin, Z., Tew, K.D. & Ronai, Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene 18, 6104–6111 (1999).
Buttke, T.M. & Sandstrom, P.A. Redox regulation of programmed cell death in lymphocytes. Free Radical Res. 22, 389–397 (1995).
Roth, S. & Droge, W. Regulation of T-cell activation and T-cell growth factor (TCGF) production by hydrogen peroxide. Cell Immunol. 108, 417–424 (1987).
Staal, F.J., Anderson, M.T., Staal, G.E., Herzenberg, L.A. & Gitler, C. Redox regulation of signal transduction: tyrosine phosphorylation and calcium influx. Proc. Natl. Acad. Sci. USA 91, 3619–3622 (1994).
Bae, Y.S. et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 217–221 (1997).
Mahadev, K., Zilbering, A., Zhu, L. & Goldstein, B.J. Insulin-stimulated hydrogen peroxide reversibly inhibits protein- tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J. Biol. Chem. 276, 21938–21942 (2001).
Rhee, S.G., Bae, Y.S., Lee, S.R. & Kwon, J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE 53, 1–6 (2000).
Gamaley, I.A. & Klyubin, I.V. Roles of reactive oxygen species: signaling and regulation of cellular functions. Int. Rev. Cytol. 188, 203–255 (1999).
Finkel, T. Signal transduction by reactive oxygen species in non-phagocytic cells. J. Leukoc. Biol. 65, 337–340 (1999).
Gulati, P. et al. Redox regulation in mammalian signal transduction. IUBMB Life 52, 25–28 (2001).
Finkel, T. Reactive oxygen species and signal transduction. IUBMB Life 52, 3–6 (2001).
Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 (2002).
Bogdan, C. Nitric oxide and the immune response. Nature Immunol. 2, 907–916 (2001).
Bootman, M.D. et al. Calcium signaling–an overview. Semin. Cell Dev. Biol. 12, 3–10 (2001).
Huyer, G. et al. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem. 272, 843–851 (1997).
Nordberg, J. & Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biol. Med. 31, 1287–1312 (2001).
Sun, Y. & Oberley, L.W. Redox regulation of transcriptional activators. Free Radical Biol. Med. 21, 335–348 (1996).
Schreck, R., Rieber, P. & Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-κB transcription factor and HIV-1. EMBO J. 10, 2247–2258 (1991).
Caselli, A. et al. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2 . J. Biol. Chem. 273, 32554–32560 (1998).
Xu, D., Rovira, I.I., II & Finkel, T. Oxidants painting the cysteine chapel: redox regulation of PTPs. Dev. Cell 2, 251–252 (2002).
Neel, B.G. & Tonks, N.K. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell. Biol. 9, 193–204 (1997).
Andersen, J.N. et al. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol. Cell. Biol. 21, 7117–7136 (2001).
Segal, A.W. & Shatwell, K.P. The NADPH oxidase of phagocytic leukocytes. Ann. NY Acad. Sci. 832, 215–222 (1997).
Babior, B.M., Lambeth, J.D. & Nauseef, W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 397, 342–344 (2002).
Diebold, B.A. & Bokoch, G.M. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nature Immunol. 2, 211–215 (2001).
Reeves, E.P. et al. Direct interaction between p47phox and protein kinase C: evidence for targeting of protein kinase C by p47phox in neutrophils. Biochem. J. 344, 859–866 (1999).
Tsunawaki, S. & Yoshikawa, K. Relationships of p40phox with p67phox in the activation and expression of the human respiratory burst NADPH oxidase. J. Biochem. (Tokyo) 128, 777–783 (2000).
Dekaris, I., Marotti, T., Sprong, R.C., van Oirschot, J.F. & van Asbeck, B.S. Hydrogen peroxide modulation of the superoxide anion production by stimulated neutrophils. Immunopharmacol. Immunotoxicol. 20, 103–117 (1998).
Inanami, O. et al. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47phox on serine 303 or 304. J. Biol. Chem. 273, 9539–9543 (1998).
Verhoeven, A.J. The NADPH oxidase: lessons from chronic granulomatous disease neutrophils. Ann. NY Acad. Sci. 832, 85–92 (1997).
Jackson, S.H., Gallin, J.I. & Holland, S.M. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182, 751–758 (1995).
Pollock, J.D. et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nature Genet. 9, 202–209 (1995).
Roy, A. et al. Mice lacking in gp91phox subunit of NAD(P)H oxidase showed glomus cell [Ca2+](i) and respiratory responses to hypoxia. Brain Res. 872, 188–193 (2000).
De Deken, X. et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 275, 23227–23233 (2000).
Lassegue, B. et al. Novel gp91phox homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 88, 888–894 (2001).
Sorescu, D. et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation 105, 1429–1435 (2002).
Cheng, G., Cao, Z., Xu, X., van Meir, E.G. & Lambeth, J.D. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269, 131–140 (2001).
Yang, S., Madyastha, P., Bingel, S., Ries, W. & Key, L. A new superoxide-generating oxidase in murine osteoclasts. J. Biol. Chem. 276, 5452–5458 (2001).
Devadas, S., Zaritskaya, L., Rhee, S.G., Oberley, L. & Williams, M.S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 195, 59–70 (2002).
Amital, H., Tur-Kaspa, I., Tashma, Z., Hendler, I. & Shoenfeld, Y. Catalytic antibodies. Structure and possible applications. Meth. Mol. Biol. 51, 203–210 (1995).
Wentworth, P. Jr. & Janda, K.D. Catalytic antibodies: structure and function. Cell Biochem. Biophys. 35, 63–87 (2001).
Wentworth, P. Jr. et al. Antibody catalysis of the oxidation of water. Science 293, 1806–1811 (2001).
Datta, D., Vaidehi, N., Xu, X. & Goddard, W.A. 3rd. Mechanism for antibody catalysis of the oxidation of water by singlet dioxygen. Proc. Natl. Acad. Sci. USA 99, 2636–2641 (2002).
Wentworth, A.D., Jones, L.H., Wentworth, P. Jr., Janda, K.D. & Lerner, R.A. Antibodies have the intrinsic capacity to destroy antigens. Proc. Natl. Acad. Sci. USA 97, 10930–10935 (2000).
Wienands, J., Larbolette, O. & Reth, M. Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc. Natl. Acad. Sci. USA 93, 7865–7870 (1996).
Barford, D. & Neel, B.G. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 6, 249–254 (1998).
Weiss, A. & Schlessinger, J. Switching signals on or off by receptor dimerization. Cell 94, 277–280 (1998).
Meng, T.C., Fukada, T. & Tonks, N.K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9, 387–399 (2002).
Uckun, F.M. et al. Ionizing radiation stimulates unidentified tyrosine-specific protein kinases in human B-lymphocyte precursors, triggering apoptosis and clonogenic cell death. Proc. Natl. Acad. Sci. USA 89, 9005–9009 (1992).
Schieven, G.L., Kirihara, J.M., Myers, D.E., Ledbetter, J.A. & Uckun, F.M. Reactive oxygen intermediates activate NF-κB in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood 82, 1212–1220 (1993).
Zhang, Y., Wienands, J., Zurn, C. & Reth, M. Induction of the antigen receptor expression on B lymphocytes results in rapid competence for signaling of SLP-65 and Syk. EMBO J. 17, 7304–7310 (1998).
Kurosaki, T. Molecular dissection of B cell antigen receptor signaling. Bioorg. Medicinal Chem. Lett. 1, 515–527 (1998).
Schamel, W.W.A. & Reth, M. Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity 13, 5–14 (2000).
Reth, M. Antigen receptor tail clue. Nature 338, 383–384 (1989).
Cambier, J.C. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL). Immunol. Today 16, 110 (1995).
Kurosaki, T. Genetic analysis of B cell antigen receptor signaling. Annu. Rev. Immunol. 17, 555–592 (1999).
Tamir, I., Dal Porto, J.M. & Cambier, J.C. Cytoplasmic protein tyrosine phosphatases SHP-1 and SHP-2: regulators of B cell signal transduction. Curr. Opin. Immunol. 12, 307–315 (2000).
Shiue, L., Zoller, M.J. & Brugge, J.S. Syk is activated by phosphotyrosine-containing peptides representing the tyrosine-based activation motifs of the high affinity receptor for IgE. J. Biol. Chem. 270, 10498–10502 (1995).
Rowley, R.B., Burkhardt, A.L., Chao, H.G., Matsueda, G.R. & Bolen, J.B. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Igα/Igβ immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem. 270, 11590–11594 (1995).
Futterer, K., Wong, J., Grucza, R.A., Chan, A.C. & Waksman, G. Structural basis for Syk tyrosine kinase ubiquity in signal transduction pathways revealed by the crystal structure of its regulatory SH2 domains bound to a dually phosphorylated ITAM peptide. J. Mol. Biol. 281, 523–537 (1998).
Healy, J.I. & Goodnow, C.C. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 16, 645–670 (1998).
Pierce, S.K. Lipid rafts and B-cell activation. Nature Rev. Immunol. 2, 96–105 (2002).
Matko, J. & Szollosi, J. Landing of immune receptors and signal proteins on lipid rafts: a safe way to be spatio-temporally coordinated? Immunol. Lett. 82, 3–15 (2002).
Furukawa, K. et al. B lymphoblasts show oxidase activity in response to cross-linking of surface IgM and HLA-DR. Scand. J. Immunol. 35, 561–567 (1992).
Verveer, P.J., Wouters, F.S., Reynolds, A.R. & Bastiaens, P.I. Quantitative imaging of lateral ErbB1 receptor signal propagation in the plasma membrane. Science 290, 1567–1570 (2000).
Goitsuka, R. et al. BASH, a novel signaling molecule preferentially expressed in B cells of the Bursa of Fabricius. J. Immunol. 161, 5804–5808 (1998).
Wienands, J. et al. SLP-65: A new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J. Exp. Med. 188, 791–795 (1998).
Fu, C., Turck, C.W., Kurosaki, T. & Chan, A.C. BLNK: A central linker protein in B cell activation. Immunity 9, 93–103 (1998).
DeLeo, F.R. et al. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Invest. 101, 455–463 (1998).
Kawahara, T. et al. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. J. Med. Invest. 48, 190–197 (2001).
Jumaa, H. et al. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity 11, 547–554 (1999).
Bromley, S.K. et al. The immunological synapse. Annu. Rev. Immunol. 19, 375–396 (2001).
Batista, F.D., Iber, D. & Neuberger, M.S. B cells acquire antigen from target cells after synapse formation. Nature 411, 489–494 (2001).
Rutault, K., Alderman, C., Chain, B.M. & Katz, D.R. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radical Biol. Med. 26, 232–238 (1999).
Reeves, E.P. et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416, 291–297 (2002).
Bokoch, G.M. Microbial killing: hold the bleach and pass the salt! Nature Immunol. 3, 340–342 (2002).
Zola, H. The development of antibody responses in the infant. Immunol. Cell. Biol. 75, 587–590 (1997).
van der Veen, R.C. et al. Superoxide prevents nitric oxide-mediated suppression of helper T lymphocytes: decreased autoimmune encephalomyelitis in nicotinamide adenine dinucleotide phosphate oxidase knockout mice. J. Immunol. 164, 5177–5183 (2000).
Peterhans, E. Reactive oxygen species and nitric oxide in viral diseases. Biol. Trace Elem. Res. 56, 107–116 (1997).
Buffinton, G.D., Christen, S., Peterhans, E. & Stocker, R. Oxidative stress in lungs of mice infected with influenza A virus. Free Radical Res. Commun. 16, 99–110 (1992).
Colamussi, M.L., White, M.R., Crouch, E. & Hartshorn, K.L. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood 93, 2395–2403 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reth, M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol 3, 1129–1134 (2002). https://doi.org/10.1038/ni1202-1129
Issue Date:
DOI: https://doi.org/10.1038/ni1202-1129
This article is cited by
-
Evaluation of the effects of photodynamic therapy consisted of the blue laser and zinc oxide QDs on MDA-MB-231 cancer cells by inhibiting cancer markers and inducing apoptosis
Lasers in Medical Science (2024)
-
The role of ROS in tumour development and progression
Nature Reviews Cancer (2022)
-
Supplying the trip to antibody production—nutrients, signaling, and the programming of cellular metabolism in the mature B lineage
Cellular & Molecular Immunology (2022)
-
Clinical value and molecular mechanism of AQGPs in different tumors
Medical Oncology (2022)
-
The role of reactive oxygen species in the immunity induced by nano-pulse stimulation
Scientific Reports (2021)