Abstract
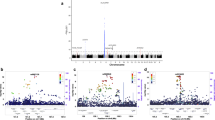
To identify new genetic risk factors for rheumatoid arthritis, we conducted a genome-wide association study meta-analysis of 5,539 autoantibody-positive individuals with rheumatoid arthritis (cases) and 20,169 controls of European descent, followed by replication in an independent set of 6,768 rheumatoid arthritis cases and 8,806 controls. Of 34 SNPs selected for replication, 7 new rheumatoid arthritis risk alleles were identified at genome-wide significance (P < 5 × 10−8) in an analysis of all 41,282 samples. The associated SNPs are near genes of known immune function, including IL6ST, SPRED2, RBPJ, CCR6, IRF5 and PXK. We also refined associations at two established rheumatoid arthritis risk loci (IL2RA and CCL21) and confirmed the association at AFF3. These new associations bring the total number of confirmed rheumatoid arthritis risk loci to 31 among individuals of European ancestry. An additional 11 SNPs replicated at P < 0.05, many of which are validated autoimmune risk alleles, suggesting that most represent genuine rheumatoid arthritis risk alleles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Silman, A.J. & Pearson, J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 4 Suppl 3, S265–S272 (2002).
Klareskog, L., Catrina, A.I. & Paget, S. Rheumatoid arthritis. Lancet 373, 659–672 (2009).
Suzuki, A. et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat. Genet. 34, 395–402 (2003).
Begovich, A.B. et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 75, 330–337 (2004).
Kurreeman, F.A. et al. A candidate gene approach identifies the TRAF1/C5 region as a risk factor for rheumatoid arthritis. PLoS Med. 4, e278 (2007).
Plenge, R.M. et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat. Genet. 39, 1477–1482 (2007).
Plenge, R.M. et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N. Engl. J. Med. 357, 1199–1209 (2007).
Remmers, E.F. et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 357, 977–986 (2007).
Thomson, W. et al. Rheumatoid arthritis association at 6q23. Nat. Genet. 39, 1431–1433 (2007).
Zhernakova, A. et al. Novel association in chromosome 4q27 region with rheumatoid arthritis and confirmation of type 1 diabetes point to a general risk locus for autoimmune diseases. Am. J. Hum. Genet. 81, 1284–1288 (2007).
Raychaudhuri, S. et al. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat. Genet. 40, 1216–1223 (2008).
Raychaudhuri, S. et al. Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat. Genet. 41, 1313–1318 (2009).
Gregersen, P.K. et al. REL, encoding a member of the NF-κB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat. Genet. 41, 820–823 (2009).
Barton, A. et al. Identification of AF4/FMR2 family, member 3 (AFF3) as a novel rheumatoid arthritis susceptibility locus and confirmation of two further pan-autoimmune susceptibility genes. Hum. Mol. Genet. 18, 2518–2522 (2009).
Barton, A. et al. Rheumatoid arthritis susceptibility loci at chromosomes 10p15, 12q13 and 22q13. Nat. Genet. 40, 1156–1159 (2008).
Marchini, J., Howie, B., Myers, S., McVean, G. & Donnelly, P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat. Genet. 39, 906–913 (2007).
Devlin, B. & Roeder, K. Genomic control for association studies. Biometrics 55, 997–1004 (1999).
de Bakker, P.I. et al. Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum. Mol. Genet. 17, R122–R128 (2008).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
Suzuki, A. et al. Functional SNPs in CD244 increase the risk of rheumatoid arthritis in a Japanese population. Nat. Genet. 40, 1224–1229 (2008).
Kochi, Y. et al. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat. Genet. 37, 478–485 (2005).
Raychaudhuri, S. et al. Identifying relationships among genomic disease regions: predicting genes at pathogenic SNP associations and rare deletions. PLoS Genet. 5, e1000534 (2009).
Barrett, J.C. et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat. Genet. 41, 703–707 (2009).
Barrett, J.C. et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat. Genet. 40, 955–962 (2008).
Harley, J.B. et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 40, 204–210 (2008).
Nair, R.P. et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat. Genet. 41, 199–204 (2009).
de Cid, R. et al. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat. Genet. 41, 211–215 (2009).
Hom, G. et al. Association of systemic lupus erythematosus with C8orf13-BEK and ITGAM-ITGAX. N. Engl. J. Med. 358, 900–909 (2008).
van Heel, D.A. et al. A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat. Genet. 39, 827–829 (2007).
Hunt, K.A. et al. Newly identified genetic risk variants for celiac disease related to the immune response. Nat. Genet. 40, 395–402 (2008).
De Jager, P.L. et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat. Genet. 41, 776–782 (2009).
Nobuhisa, I. et al. Spred-2 suppresses aorta-gonad-mesonephros hematopoiesis by inhibiting MAP kinase activation. J. Exp. Med. 199, 737–742 (2004).
Ernst, M. & Jenkins, B.J. Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet. 20, 23–32 (2004).
Graham, R.R. et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat. Genet. 38, 550–555 (2006).
Sigurdsson, S. et al. Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am. J. Hum. Genet. 76, 528–537 (2005).
Graham, R.R. et al. Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc. Natl. Acad. Sci. USA 104, 6758–6763 (2007).
Sigurdsson, S. et al. Association of a haplotype in the promoter region of the interferon regulatory factor 5 gene with rheumatoid arthritis. Arthritis Rheum. 56, 2202–2210 (2007).
Dendrou, C.A. et al. Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat. Genet. 41, 1011–1015 (2009).
Maier, L.M. et al. IL2RA genetic heterogeneity in multiple sclerosis and type 1 diabetes susceptibility and soluble interleukin-2 receptor production. PLoS Genet. 5, e1000322 (2009).
Todd, J.A. et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat. Genet. 39, 857–864 (2007).
Zhernakova, A., van Diemen, C.C. & Wijmenga, C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat. Rev. Genet. 10, 43–55 (2009).
Plenge, R.M. Recent progress in rheumatoid arthritis genetics: one step towards improved patient care. Curr. Opin. Rheumatol. 21, 262–271 (2009).
Rothenberg, E.V., Moore, J.E. & Yui, M.A. Launching the T-cell-lineage developmental programme. Nat. Rev. Immunol. 8, 9–21 (2008).
Hirota, K. et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 204, 2803–2812 (2007).
Wucherpfennig, K.W., Call, M.J., Deng, L. & Mariuzza, R. Structural alterations in peptide-MHC recognition by self-reactive T cell receptors. Curr. Opin. Immunol. 21, 590–595 (2009).
Sakaguchi, N. et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426, 454–460 (2003).
MacGregor, A.J. et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum. 43, 30–37 (2000).
van der Woude, D. et al. Quantitative heritability of anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis. Arthritis Rheum. 60, 916–923 (2009).
Arnett, F.C. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31, 315–324 (1988).
Neale, B.M. et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc. Natl. Acad. Sci. USA 107, 7395–7400 (2010).
Kathiresan, S. et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 41, 334–341 (2009).
Coenen, M.J. et al. Common and different genetic background for rheumatoid arthritis and coeliac disease. Hum. Mol. Genet. 18, 4195–4203 (2009).
Wijbrandts, C.A. et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann. Rheum. Dis. 67, 1139–1144 (2008).
Costenbader, K.H., Chang, S.C., De Vivo, I., Plenge, R. & Karlson, E.W. PTPN22, PADI4 and CTLA4 genetic polymorphisms and risk of rheumatoid arthritis in two longitudinal cohort studies: evidence of gene-environment interactions with heavy cigarette smoking. Arthritis Res. Ther. 10, R52 (2008).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Gateva, V. et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat. Genet. 41, 1228–1233 (2009).
Acknowledgements
R.M.P. is supported by grants from the US National Institutes of Health (NIH) (R01-AR057108, R01-AR056768 and U54 RR020278), a private donation from the Fox Trot Fund, the William Randolph Hearst Fund of Harvard University, the American College of Rheumatology 'Within Our Reach' campaign and a Career Award for Medical Scientists from the Burroughs Wellcome Fund. S.R. is supported by an NIH Career Development Award (1K08AR055688-01A1) and an American College of Rheumatology Bridge Grant. F.A.S.K. is supported by an EMBO-UNESCO L'Oreal Fellowship. The Broad Institute Center for Genotyping and Analysis is supported by grant U54 RR020278 from the National Center for Research Resources. The BRASS Registry is supported by a grant from Crescendo and Biogen-Idec. EIRA is supported by grants from the Swedish Medical Research council, the Swedish Council for Working Life and Social Research, King Gustaf V's 80-year foundation, the Swedish Rheumatism Foundation, Stockholm County Council, from Vinnova and the insurance company AFA. NARAC is supported by the NIH (NO1-AR-2-2263 and RO1 AR44422). L.A.C. is supported by the NIH (R01 AI065841 and 5-M01-RR-00079). The Nurses Health Study is supported by NIH grants P01 CA87969, CA49449, CA67262, CA50385, AR049880-06 and AR47782. This research was also supported in part by the Intramural Research Program of the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the US National Institutes of Health. This research was also supported in part by grants to K.A.S. from the Canadian Institutes for Health Research (MOP79321 and IIN-84042) and the Ontario Research Fund (RE01061) and by a Canada Research Chair. Genotyping of United Kingdom Rheumatoid Arthritis Genetics samples was supported by the Arthritis Research campaign arc grant reference number 17552 and by the Manchester Biomedical Research Centre and Manchester Academy of Health Sciences. C.W. was funded by the Netherlands Organization for Scientific Research (VICI grant 918.66.620). We acknowledge the help of B.A.C. Dijkmans, D. van Schaardenburg, A. Salvador Peña, P.L. Klarenbeek, Z. Zhang, M.T. Nurmohamed, W.F. Lems, R.R. J. van de Stadt, W.H. Bos, J. Ursum, M.G.M. Bartelds, D.M. Gerlag, M.G.H. van der Sande, C.A. Wijbrandts and M.M.J. Herenius in gathering Genetics Network Rheumatology Amsterdam subject samples and data. We thank the Myocardial Infarction Genetics Consortium (MIGen) study for the use of genotype data from their healthy controls in our study. The MIGen study was funded by the US National Institutes of Health and National Heart, Lung, and Blood Institute's SNP Typing for Association with Multiple Phenotypes from Existing Epidemiological Data (STAMPEED) genomics research program R01HL087676 and a grant from the National Center for Research Resources. We thank J. Seddon, Progression of AMD Study, Age-Related Macular Degeneration (AMD) Registry Study, Family Study of AMD, The US Twin Study of AMD and the Age-Related Eye Disease Study (AREDS) for use of genotype data from their healthy controls in our study. We thank D. Hafler and the Multiple Sclerosis Collaborative for use of genotype data from their healthy controls recruited at Brigham and Women's Hospital.
Author information
Authors and Affiliations
Consortia
Contributions
Study design: R.M.P., S.R., E.A.S. Analysis: E.A.S. (lead), S.R., F.A.S.K., R.C. Sample procurement and data generation: J.W., K.A.S., P.K.G., L.K., N.A.S., M.E.W., C.W., M.J.H.C., N.d.V., P.P.T., E.W.K., R.E.M.T., T.W.J.H., A.B.B. (leads); E.F.R., G.X., S.E., B.P.T., Y.L., A.Z., A.H., C.G., L.A., C.I.A., K.G.A., A.B., J.B., E.B., N.P.B., J.J.C., J. Coblyn, K.H.C., L.A.C., J.B.A.C., J. Cui, P.I.W.d.B., P.L.D.J., B.D., P.E., E.F., P.H., L.J.H., D.L.K., X.K., A.T.L., X.L., P.M., A.W.M., L.P., M.D.P., T.R.D.J.R., D.M.R., M.S., M.F.S., S.S., W.T., A.H.M.v.d.H.-v.M., I.E.v.d.H.-B., C.E.v.d.S., P.L.C.M.v.R., A.G.W., G.J.W., B.P.W., BIRAC and YEAR consortia. Writing: R.M.P., E.A.S. (leads); S.R., F.A.S.K. (primary contributors); J.W., K.A.S., P.K.G., L.K., N.A.S., M.E.W., C.W., M.J.H.C., N.d.V., P.P.T., E.W.K., R.E.M.T., T.W.J.H., A.B.B., E.F.R., G.X., S.E., B.P.T., Y.L., A.Z., A.H., C.G., L.A., C.I.A., K.G.A., A.B., J.B., E.B., N.P.B., J.J.C., J. Coblyn, K.H.C., L.A.C., J.B.A.C., J. Cui, P.I.W.d.B., P.L.D.J., B.D., P.E., E.F., P.H., L.J.H., D.L.K., X.K., A.T.L., X.L., P.M., A.W.M., L.P., M.D.P., T.R.D.J.R., D.M.R., M.S., M.F.S., S.S., W.T., A.H.M.v.d.H.-v.M., I.E.v.d.H.-B., C.E.v.d.S., P.L.C.M.v.R., A.G.W., G.J.W., B.P.W.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A full list of members is provided in the Supplementary Note.
A full list of members is provided in the Supplementary Note.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Tables 1–6 and Supplementary Figures 1–7 (PDF 14716 kb)
Rights and permissions
About this article
Cite this article
Stahl, E., Raychaudhuri, S., Remmers, E. et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 42, 508–514 (2010). https://doi.org/10.1038/ng.582
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.582
This article is cited by
-
Loss of AR-regulated AFF3 contributes to prostate cancer progression and reduces ferroptosis sensitivity by downregulating ACSL4 based on single-cell sequencing analysis
Apoptosis (2024)
-
Genome-wide identification of RNA modification-related single nucleotide polymorphisms associated with rheumatoid arthritis
BMC Genomics (2023)
-
Integrative Mendelian randomization reveals the soluble receptor for advanced glycation end products as protective in relation to rheumatoid arthritis
Scientific Reports (2023)
-
Genetic variants of interferon-response factor 5 are associated with the incidence of chronic kidney disease: the D.E.S.I.R. study
Genes & Immunity (2023)
-
Genome-wide exploratory analysis for NARAC dataset with preparation for haplotype block partitioning through minor allele frequency quality control viewpoint
Iran Journal of Computer Science (2023)