Abstract
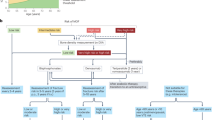
Osteoporosis is a major public health problem that is characterized by microarchitectural deterioration, low bone mass, and increased risk of fractures. Currently, many women and men affected with this disease are not diagnosed or treated. As osteoporosis is often clinically silent, risk-factor assessment and measurement of BMD are needed to identify those who may benefit from osteoporosis therapy. Although adequate daily intake of calcium and vitamin D, and regular weight-bearing exercise are important for skeletal health, they are not adequate treatments for individuals with osteoporosis. Therapies approved for treatment and/or prevention of osteoporosis in the United States include oral bisphosphonates (alendronate, ibandronate and risedronate), calcitonin, estrogens, teriparatide (parathyroid hormone fragment [1–34]), and raloxifene. For most patients, oral bisphosphonates are the treatment of choice, given the large-scale randomized-trial data demonstrating efficacy in fracture reduction, although bisphosphonates that reduce spine and nonspine fractures (e.g. alendronate and risedronate) are preferred. For high-risk patients (those with very low bone density, or with fractures), teriparatide therapy for 2 years should be considered. The treatment paradigm for osteoporosis will evolve further as promising new treatments progress through clinical development.
Key Points
-
Osteoporosis is a common disease in aging women and men, and it is a significant contributing factor to more than 1.5 million fractures of the spine, hip, and forearm each year in the United States
-
Initial therapy includes adequate calcium, vitamin D, and weight-bearing exercise; vitamin D insufficiency is common, and many investigators believe that the minimum 25-hydroxyvitamin D level necessary to reduce fracture risk clusters is in the range 70–80 nmol/l (28–32 ng/ml)
-
Although estrogen replacement therapy is effective in preventing fracture, it is no longer recommended for long-term treatment of osteoporosis
-
The oral bisphosphonates are the most widely used antiresorptive therapies for the treatment of osteoporosis: alendronate and risedronate decrease risk of vertebral and nonvertebral fractures, but the ideal duration of bisphosphonate use is uncertain
-
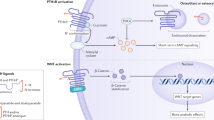
Parathyroid hormone administered intermittently improves skeletal microarchitecture, improves bone mass, and decreases vertebral and nonvertebral fractures
-
Several new and promising osteoporosis therapies are under investigation and the management of osteoporosis will continue to evolve as emerging therapies become available; osteoporosis therapy should be individualized, weighing fracture risk, therapy benefits, and side effects
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
United States Surgeon General (2004) Surgeon General's Report on Osteoporosis and Bone Health for the American Society of Bone and Mineral Research, Part Two, Chapter 4 (The Frequency of Bone Disease) [http://www.surgeongeneral.gov/library/bonehealth/chapter_4.html] (accessed 10 July 2006)
National Committee for Quality Assurance (2005) The State of Healthcare Quality 2005: Industry Trends and Analysis. National Committee for Quality Assurance Health Plan Employer Data and Information Set, page 48 [http://www.ncqa.org/] (accessed 10 July 2006)
Holick MF et al. (2005) Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90: 3215–3224
LeBoff MS et al. (1999) Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 281: 1505–1511
Glowacki J et al. (2003) Osteoporosis and vitamin-D deficiency among postmenopausal women with osteoarthritis undergoing total hip arthroplasty. J Bone Joint Surg Am 85: 2371–2377
Thomas MK et al. (1998) Hypovitaminosis D in medical inpatients. N Engl J Med 338: 777–783
Chapuy MC et al. (1992) Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med 327: 1637–1642
Dawson-Hughes B et al. (1997) Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med 337: 670–676
Trivedi DP et al. (2003) Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 326: 469
Shaukat A et al. (2005) Role of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trials. Am J Gastroenterol 100: 390–394
Dawson-Hughes B et al. (2005) Estimates of optimal vitamin D status. Osteoporos Int 16: 713–716
Jackson RD et al. (2006) Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354: 669–683
Grant AM et al. (2005) Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 365: 1621–1628
Heaney RP et al. (2003) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77: 204–210
Vieth R et al. (2004) Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 µg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J 3: 8
Binkley N et al. (2004) Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab 89: 3152–3157
Institute of Medicine (1997) Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: The National Academies Press
Manolagas SC and Jilka RL (1992) Cytokines, hematopoiesis, osteoclastogenesis, and estrogens. Calcif Tissue Int 50: 199–202
Pacifici R et al. (1989) Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci USA 86: 2398–2402
Rossouw JE et al. (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288: 321–333
Ettinger B et al. (2004) Effects of ultralow-dose transdermal estradiol on bone mineral density: a randomized clinical trial. Obstet Gynecol 104: 443–451
Bagger Y et al. (2004) Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: the PERF study. Bone 34: 728–735
Rogers MJ (2004) From molds and macrophages to mevalonate: a decade of progress in understanding the molecular mode of action of bisphosphonates. Calcif Tissue Int 75: 451–461
Boivin GY et al. (2000) Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 27: 687–694
Black DM et al. (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348: 1535–1541
Cummings SR et al. (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280: 2077–2082
Liberman UA et al. (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333: 1437–1443
Orwoll E et al. (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343: 604–610
Adachi JD et al. (2001) Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 44: 202–211
Hosking D et al. (1998) Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med 338: 485–492
Rizzoli R et al. (2002) Two-year results of once-weekly administration of alendronate 70 mg for the treatment of postmenopausal osteoporosis. J Bone Miner Res 17: 1988–1996
Ott SM (2005) Long-term safety of bisphosphonates. J Clin Endocrinol Metab 90: 1897–1899
Bone HG et al. (2004) Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350: 1189–1199
Chavassieux PM et al. (1997) Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest 100: 1475–1480
Black D et al. (2004) A 5 year randomized trial of the long-term efficacy and safety of alendronate: The FIT Long-term EXtension (FLEX). J Bone Miner Res 19: S45
Ensrud KE et al. (2004) Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res 19: 1259–1269
Harris ST et al. (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282: 1344–1352
McClung MR et al. (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344: 333–340
Reginster J et al. (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11: 83–91
Brown JP et al. (2002) The efficacy and tolerability of risedronate once a week for the treatment of postmenopausal osteoporosis. Calcif Tissue Int 71: 103–111
Mellstrom DD et al. (2004) Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 75: 462–468
Cohen S et al. (1999) Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 42: 2309–2318
Rosen CJ et al. (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20: 141–151
Rosen CJ (2005) Clinical practice. Postmenopausal osteoporosis. N Engl J Med 353: 595–603
Chesnut CH III et al. (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19: 1241–1249
Vis M et al. (2005) The effect of intravenous pamidronate versus oral alendronate on bone mineral density in patients with osteoporosis. Osteoporos Int 16: 1432–1435
Reid IR et al. (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346: 653–661
Woo SB et al. (2006) Systematic review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med 144: 753–761
Neer RM et al. (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344: 1434–1441
Lindsay R et al. (1997) Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350: 550–555
Orwoll ES et al. (2003) The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J Bone Miner Res 18: 9–17
Kurland ES et al. (2000) Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85: 3069–3076
Lane NE et al. (1998) Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest 102: 1627–1633
Body JJ et al. (2002) A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1–34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 87: 4528–4535
Hodsman AB et al. (2003) Efficacy and safety of human parathyroid hormone-(1–84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab 88: 5212–5220
Cosman F et al. (2005) Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 353: 566–575
Dempster DW et al. (2001) Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 16: 1846–1853
Jiang Y et al. (2003) Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res 18: 1932–1941
Zanchetta JR et al. (2003) Effects of teriparatide [recombinant human parathyroid hormone (1–34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res 18: 539–543
Horwitz MJ et al. (2003) Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 88: 569–575
Hodsman AB et al. (2005) Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26: 688–703
Vahle JL et al. (2002) Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol 30: 312–321
Ettinger B et al. (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282: 637–645
Delmas PD et al. (2002) Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab 87: 3609–3617
Siris ES et al. (2005) Skeletal effects of raloxifene after 8 years: results from the continuing outcomes relevant to Evista (CORE) study. J Bone Miner Res 20: 1514–1524
Chesnut CH III et al. (2000) A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med 109: 267–276
Tanko LB et al. (2004) Safety and efficacy of a novel salmon calcitonin (sCT) technology-based oral formulation in healthy postmenopausal women: acute and 3-month effects on biomarkers of bone turnover. J Bone Miner Res 19: 1531–1538
Black DM et al. (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349: 1207–1215
Finkelstein JS et al. (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349: 1216–1226
Deal C et al. (2005) Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res 20: 1905–1911
Rittmaster RS et al. (2000) Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 85: 2129–2134
Kurland ES et al. (2004) The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone(1–34)]. Osteoporos Int 15: 992–997
Dahl SG et al. (2001) Incorporation and distribution of strontium in bone. Bone 28: 446–453
Canalis E et al. (1996) The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 18: 517–523
Baron R and Tsouderos Y (2002) In vitro effects of S12911-2 on osteoclast function and bone marrow macrophage differentiation. Eur J Pharmacol 450: 11–17
Marie PJ et al. (1993) An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J Bone Miner Res 8: 607–615
Nielsen SP et al. (1999) Influence of strontium on bone mineral density and bone mineral content measurements by dual X-ray absorptiometry. J Clin Densitom 2: 371–379
Meunier PJ et al. (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350: 459–468
Reginster JY et al. (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90: 2816–2822
Bekker PJ et al. (2001) The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 16: 348–360
Cundy T et al. (2005) Recombinant osteoprotegerin for juvenile Paget's disease. N Engl J Med 353: 918–923
McClung M et al. (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354: 821–831
Arey BJ et al. (2005) A novel calcium-sensing receptor antagonist transiently stimulates parathyroid hormone secretion in vivo. Endocrinology 146: 2015–2022
Warmington K et al. (2005) Sclerostin monoclonal antibody treatment of osteoporotic rats completely reverses one year of ovariectomy-induced systemic bone loss. J Bone Miner Res 20 (Suppl 1): S22–1082
Murphy MG et al. (2005) Effect of L-000845704, an αVβ3 integrin antagonist, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab 90: 2022–2028
Tavares FX et al. (2004) Design of potent, selective, and orally bioavailable inhibitors of cysteine protease cathepsin K. J Med Chem 47: 588–599
Anderson GL et al. (2004) Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA 291: 1701–1712
Hulley SB and Grady D (2004) The WHI estrogen-alone trial—do things look any better? JAMA 291: 1769–1771
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
MS LeBoff declared associations with the following companies: Amgen (stock ownership), Eli Lilly (research grant), Novartis (consultant, research grant), NPS Allelix (research grant), and Procter & Gamble (advisory board). The other authors declared they have no competing interests.
Rights and permissions
About this article
Cite this article
Mulder, J., Kolatkar, N. & LeBoff, M. Drug Insight: existing and emerging therapies for osteoporosis. Nat Rev Endocrinol 2, 670–680 (2006). https://doi.org/10.1038/ncpendmet0325
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpendmet0325
This article is cited by
-
Structure of the glucagon receptor in complex with a glucagon analogue
Nature (2018)
-
Artemisia annua extract prevents ovariectomy-induced bone loss by blocking receptor activator of nuclear factor kappa-B ligand-induced differentiation of osteoclasts
Scientific Reports (2017)
-
Stimulation of Osteogenic Differentiation by Saikosaponin-A in Bone Marrow Stromal Cells Via WNT/β-Catenin Pathway
Calcified Tissue International (2017)
-
Comparison of treatment effects of teriparatide and the bisphosphonate risedronate in an aged, osteopenic, ovariectomized rat model under various clinical conditions
Journal of Bone and Mineral Metabolism (2016)
-
Bisphosphonate treatment and risk of esophageal cancer: a meta-analysis of observational studies
Osteoporosis International (2013)