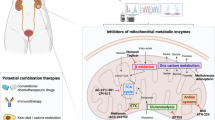
Abstract
Mitochondria have a well-recognized role in the production of ATP and the intermediates needed for macromolecule biosynthesis, such as nucleotides. Mitochondria also participate in the activation of signaling pathways. Overall, accumulating evidence now suggests that mitochondrial bioenergetics, biosynthesis and signaling are required for tumorigenesis. Thus, emerging studies have begun to demonstrate that mitochondrial metabolism is potentially a fruitful arena for cancer therapy. In this Perspective, we highlight recent developments in targeting mitochondrial metabolism for the treatment of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bonnet, S. et al. A mitochondria-K channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11, 37–51 (2007)
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956)
Ames, B.N., Shigenaga, M.K. & Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 90, 7915–7922 (1993)
Koppenol, W.H., Bounds, P.L. & Dang, C.V. Otto Warburg's contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11, 325–337 (2011)
Cross, C.E. et al. Oxygen radicals and human disease. Ann. Intern. Med. 107, 526–545 (1987)
Weinhouse, S. On respiratory impairment in cancer cells. Science 124, 267–269 (1956)
Weinhouse, S., Millington, R.H. & Wenner, C.E. Metabolism of neoplastic tissue. I. The oxidation of carbohydrate and fatty acids in transplanted tumors. Cancer Res. 11, 845–850 (1951).
Wenner, C.E., Spirtes, M.A. & Weinhouse, S. Metabolism of neoplastic tissue. II. A survey of enzymes of the citric acid cycle in transplanted tumors. Cancer Res. 12, 44–49 (1952).
Weinhouse, S. Studies on the fate of isotopically labeled metabolites in the oxidative metabolism of tumors. Cancer Res. 11, 585–591 (1951).
Weinberg, F. et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 107, 8788–8793 (2010) This report provides genetic and pharmacologic evidence that mitochondrial metabolism and ROS are required for tumor growth.
Fogal, V. et al. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol. Cell. Biol. 30, 1303–1318 (2010)
Guo, J.Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460–470 (2011) This report provides evidence that autophagy sustains mitochondrial metabolism that is essential for tumor growth.
Wellen, K.E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009)
Kaelin, W.G. Jr. & McKnight, S.L. Influence of metabolism on epigenetics and disease. Cell 153, 56–69 (2013)
Sena, L.A. & Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012)
Pagliarini, D.J. et al. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic β cells. Mol. Cell 19, 197–207 (2005)
Acin-Perez, R., Gatti, D.L., Bai, Y. & Manfredi, G. Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 13, 712–719 (2011)
Huang, L.J. et al. NH2-terminal targeting motifs direct dual specificity A-kinase-anchoring protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum. J. Cell Biol. 145, 951–959 (1999)
Seth, R.B., Sun, L., Ea, C.K. & Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kB and IRF 3. Cell 122, 669–682 (2005)
Chandel, N.S. Mitochondria as signaling organelles. BMC Biol. 12, 34 (2014)
Ward, P.S. & Thompson, C.B. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 (2012)
Lunt, S.Y. & Vander Heiden, M.G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011)
Dang, C.V. Links between metabolism and cancer. Genes Dev. 26, 877–890 (2012)
Hatzivassiliou, G. et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell 8, 311–321 (2005)
DeBerardinis, R.J. & Cheng, T. Q's next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29, 313–324 (2010)
Schieber, M. & Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462 (2014)
Gorrini, C., Harris, I.S. & Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 12, 931–947 (2013) A superb review on targeting ROS for cancer therapy.
Fan, J. et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510, 298–302 (2014)
Lewis, C.A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014) This paper reports the importance of mitochondrial one-carbon metabolism in generating mitochondrial NADPH.
Yang, M., Soga, T. & Pollard, P.J. Oncometabolites: linking altered metabolism with cancer. J. Clin. Invest. 123, 3652–3658 (2013)
Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407 (2005)
Mullen, A.R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2012).
Wise, D.R. et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. USA 108, 19611–19616 (2011)
Metallo, C.M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2012).
Sullivan, L.B. et al. The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling. Mol. Cell 51, 236–248 (2013)
Guzy, R.D., Sharma, B., Bell, E., Chandel, N. & Schumacker, P. Loss of the SdhB, but not the SdhA, subunit of complex II triggers reactive oxygen species–dependent hypoxia-inducible factor activation and tumorigenesis. Mol. Cell. Biol. 28, 718–731 (2008)
Woo, D.K. et al. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(Min/+) mice. Am. J. Pathol. 180, 24–31 (2012)
Petros, J.A. et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl. Acad. Sci. USA 102, 719–724 (2005)
Ishikawa, K. et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 (2008)
Porporato, P.E. et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 8, 754–766 (2014)
Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 12, 685–698 (2012)
Zu, X.L. & Guppy, M. Cancer metabolism: facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 313, 459–465 (2004) A report that highlights that most cancer cells generate the majority of their ATP from mitochondria.
Fan, J. et al. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 9, 712 (2013)
Jain, R.K., Munn, L.L. & Fukumura, D. Dissecting tumour pathophysiology using intravital microscopy. Nat. Rev. Cancer 2, 266–276 (2002)
Gatenby, R.A. & Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 (2004)
Rumsey, W.L., Schlosser, C., Nuutinen, E.M., Robiolio, M. & Wilson, D.F. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J. Biol. Chem. 265, 15392–15402 (1990).
Caro, P. et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell 22, 547–560 (2012)
Vazquez, F. et al. PGC1a expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell 23, 287–301 (2013)
Haq, R. et al. Oncogenic BRAF regulates oxidative metabolism via PGC1a and MITF. Cancer Cell 23, 302–315 (2013)
Engelman, J.A. et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat. Med. 14, 1351–1356 (2008)
Bailey, C.J. & Turner, R.C. Metformin. N. Engl. J. Med. 334, 574–579 (1996)
Evans, J.M., Donnelly, L.A., Emslie-Smith, A.M., Alessi, D.R. & Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. Br. Med. J. 330, 1304–1305 (2005) A seminal study that was the first to report a potential association of metformin use with reduced cancer incidence.
Dowling, R.J., Niraula, S., Stambolic, V. & Goodwin, P.J. Metformin in cancer: translational challenges. J. Mol. Endocrinol. 48, R31–R43 (2012)
Buzzai, M. et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 67, 6745–6752 (2007)
Memmott, R.M. et al. Metformin prevents tobacco carcinogen–induced lung tumorigenesis. Cancer Prev. Res. (Phila.) 3, 1066–1076 (2010)
Tomimoto, A. et al. Metformin suppresses intestinal polyp growth in ApcMin/+ mice. Cancer Sci. 99, 2136–2141 (2008)
Pollak, M. Overcoming drug development bottlenecks with repurposing: repurposing biguanides to target energy metabolism for cancer treatment. Nat. Med. 20, 591–593 (2014)
Birsoy, K., Sabatini, D.M. & Possemato, R. Untuning the tumor metabolic machine: targeting cancer metabolism: a bedside lesson. Nat. Med. 18, 1022–1023 (2012)
Pollak, M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat. Rev. Cancer 12, 159–169 (2012).
El-Mir, M.Y. et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 (2000)
Owen, M.R., Doran, E. & Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 (2000)
Bridges, H.R., Jones, A.J., Pollak, M.N. & Hirst, J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 462, 475–487 (2014) A detailed study of how metformin and related biguanides inhibit mitochondrial oxidative phosphorylation.
Wheaton, W.W. et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 (2014) A report that indicates that metformin's anti-cancer effects in vivo are due to complex I inhibition.
Fendt, S.M. et al. Metformin decreases glucose oxidation and increases the dependency of prostate cancer cells on reductive glutamine metabolism. Cancer Res. 73, 4429–4438 (2013)
Janzer, A. et al. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc. Natl. Acad. Sci. USA 111, 10574–10579 (2014)
Hardie, D.G. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes 62, 2164–2172 (2013)
Storozhuk, Y. et al. Metformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPK. Br. J. Cancer 108, 2021–2032 (2013)
Emami Riedmaier, A., Fisel, P., Nies, A.T., Schaeffeler, E. & Schwab, M. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol. Sci. 34, 126–135 (2013)
Appleyard, M.V. et al. Phenformin as prophylaxis and therapy in breast cancer xenografts. Br. J. Cancer 106, 1117–1122 (2012)
Birsoy, K. et al. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 508, 108–112 (2014) A study demonstrating that sensitivity to biguanides increases in cancer cells that harbor mutations in complex I genes or have impaired glucose utilization.
Shackelford, D.B. et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 23, 143–158 (2013)
Yuan, P. et al. Phenformin enhances the therapeutic benefit of BRAFV600E inhibition in melanoma. Proc. Natl. Acad. Sci. USA 110, 18226–18231 (2013)
Zhang, X. et al. Induction of mitochondrial dysfunction as a strategy for targeting tumour cells in metabolically compromised microenvironments. Nat Commun. 5, 3295 (2014).
Škrtić, M. et al. Inhibition of mitochondrial translation as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 20, 674–688 (2011)
Chae, Y.C. et al. Control of tumor bioenergetics and survival stress signaling by mitochondrial HSP90s. Cancer Cell 22, 331–344 (2012)
Hensley, C.T., Wasti, A.T. & DeBerardinis, R.J. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest. 123, 3678–3684 (2013) An excellent review of glutamine metabolism and its potential as a therapeutic target.
Wise, D.R. et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 105, 18782–18787 (2008)
Gao, P. et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765 (2009)
Gaglio, D. et al. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 7, 523 (2011)
Wang, J.B. et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18, 207–219 (2010)
Le, A. et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 15, 110–121 (2012)
Thornburg, J.M. et al. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 10, R84 (2008)
Qing, G. et al. ATF4 Regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 22, 631–644 (2012)
Strohecker, A.M. et al. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov. 3, 1272–1285 (2013)
Bjelakovic, G. & Gluud, C. Surviving antioxidant supplements. J. Natl. Cancer Inst. 99, 742–743 (2007)
Sayin, V.I. et al. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 6, 221ra15 (2014).
The α-Tocopherol β-Carotene Cancer Prevention Study Group. The effect of vitamin-E and b-carotene on the incidence of lung-cancer and other cancers in male smokers. N. Engl. J. Med. 330, 1029–1035 (1994)
Cheng, G. et al. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 72, 2634–2644 (2012)
Nazarewicz, R.R. et al. Does scavenging of mitochondrial superoxide attenuate cancer prosurvival signaling pathways? Antioxid. Redox Signal. 19, 344–349 (2013)
Snow, B.J. et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov. Disord. 25, 1670–1674 (2010)
DeNicola, G.M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109 (2011). The paper identifies mitochondrial one-carbon metabolic enzyme MTHFD2 as a potential cancer therapeutic target across multiple tumors.
Locasale, J.W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013)
Ye, J. et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. (2014).
Nilsson, R. et al. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat. Commun. 5, 3128 (2014)
Glasauer, A., Sena, L.A., Diebold, L.P., Mazar, A.P. & Chandel, N.S. Targeting SOD1 reduces experimental non-small-cell lung cancer. J. Clin. Invest. 124, 117–128 (2014)
Trachootham, D. et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell 10, 241–252 (2006)
Martin-Rufián, M. et al. Both GLS silencing and GLS2 overexpression synergize with oxidative stress against proliferation of glioma cells. J. Mol. Med. (Berl.) 92, 277–290 (2014)
Raj, L. et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 475, 231–234 (2011)
Fesik, S.W. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer 5, 876–885 (2005)
Murphy, M.P. & Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 47, 629–656 (2007) An excellent review that details mechanisms by which small molecules preferentially can be delivered into mitochondrial matrix.
Marrache, S., Pathak, R.K. & Dhar, S. Detouring of cisplatin to access mitochondrial genome for overcoming resistance. Proc. Natl. Acad. Sci. USA 111, 10444–10449 (2014)
Viale, A. et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 514, 628–632 (2014)
Acknowledgements
This work is supported US National Institutes of Health grants R01CA123067 to N.S.C. and 5T32HL076139-10 to S.E.W. We apologize to all investigators whose work could not be cited due to reference limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
N.S.C. has previously consulted for Agios, Astellas Pharma Inc. and Bayer Pharma AG on this topic.
Rights and permissions
About this article
Cite this article
Weinberg, S., Chandel, N. Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol 11, 9–15 (2015). https://doi.org/10.1038/nchembio.1712
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1712
This article is cited by
-
Ovarian cancer cells regulate their mitochondrial content and high mitochondrial content is associated with a poor prognosis
BMC Cancer (2024)
-
LINC00571 drives tricarboxylic acid cycle metabolism in triple-negative breast cancer through HNRNPK/ILF2/IDH2 axis
Journal of Experimental & Clinical Cancer Research (2024)
-
SIRT5-mediated ME2 desuccinylation promotes cancer growth by enhancing mitochondrial respiration
Cell Death & Differentiation (2024)
-
Preneoplastic cells switch to Warburg metabolism from their inception exposing multiple vulnerabilities for targeted elimination
Oncogenesis (2024)
-
Compensatory cross-talk between autophagy and glycolysis regulates senescence and stemness in heterogeneous glioblastoma tumor subpopulations
Acta Neuropathologica Communications (2023)