Abstract
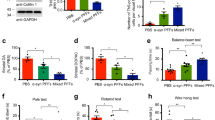
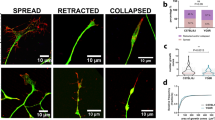
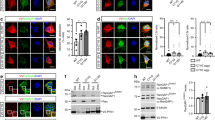
Hyperphosphorylated forms of the microtubule-associated protein (MAP) tau accumulate in Alzheimer's disease and related tauopathies and are thought to have an important role in neurodegeneration. However, the mechanisms through which phosphorylated tau induces neurodegeneration have remained elusive. Here, we show that tau-induced neurodegeneration is associated with accumulation of filamentous actin (F-actin) and the formation of actin-rich rods in Drosophila and mouse models of tauopathy. Importantly, modulating F-actin levels genetically leads to dramatic modification of tau-induced neurodegeneration. The ability of tau to interact with F-actin in vivo and in vitro provides a molecular mechanism for the observed phenotypes. Finally, we show that the Alzheimer's disease-linked human β-amyloid protein (Aβ) synergistically enhances the ability of wild-type tau to promote alterations in the actin cytoskeleton and neurodegeneration. These findings raise the possibility that a direct interaction between tau and actin may be a critical mediator of tau-induced neurotoxicity in Alzheimer's disease and related disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Buee, L., Bussiere, T., Buee-Scherrer, V., Delacourte, A. & Hof, P. R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 33, 95–130 (2000).
Lee, V. M., Goedert, M. & Trojanowski, J. Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 (2001).
Cross, D., Vial, C. & Maccioni, R. B. A tau-like protein interacts with stress fibers and microtubules in human and rodent cultured cell lines. J. Cell Sci. 105, 51–60 (1993).
DiTella, M., Feiguin, F., Morfini, G. & Caceres, A. Microfilament-associated growth cone component depends upon Tau for its intracellular localization. Cell Motil. Cytoskeleton 29, 117–130 (1994).
Henriquez, J. P., Cross, D., Vial, C. & Maccioni, R. B. Subpopulations of tau interact with microtubules and actin filaments in various cell types. Cell Biochem. Funct. 13, 239–250 (1995).
Kempf, M., Clement, A., Faissner, A., Lee, G. & Brandt, R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J. Neurosci. 16, 5583–5592 (1996).
Cunningham, C. C. et al. Microtubule-associated protein 2c reorganizes both microtubules and microfilaments into distinct cytological structures in an actin-binding protein-280-deficient melanoma cell line. J. Cell Biol. 136, 845–857 (1997).
Zmuda, J. F. & Rivas, R. J. Actin disruption alters the localization of tau in the growth cones of cerebellar granule neurons. J. Cell Sci. 113, 2797–2809 (2000).
Griffith, L. M. & Pollard, T. D. The interaction of actin filaments with microtubules and microtubule-associated proteins. J. Biol. Chem. 257, 9143–9151 (1982).
Correas, I., Padilla, R. & Avila, J. The tubulin-binding sequence of brain microtubule-associated proteins, tau and MAP-2, is also involved in actin binding. Biochem J. 269, 61–64 (1990).
Moraga, D. M., Nunez, P., Garrido, J. & Maccioni, R. B. A tau fragment containing a repetitive sequence induces bundling of actin filaments. J. Neurochem. 61, 979–986 (1993).
Farias, G. A., Munoz, J. P., Garrido, J. & Maccioni, R. B. Tubulin, actin, and tau protein interactions and the study of their macromolecular assemblies. J. Cell Biochem. 85, 315–324 (2002).
Roger, B., Al-Bassam, J., Dehmelt, L., Milligan, R. A. & Halpain, S. MAP2c, but not tau, binds and bundles F-actin via its microtubule binding domain. Curr. Biol. 14, 363–371 (2004).
Schochet, S. S. Jr., Lampert, P. W. & Lindenberg, R. Fine structure of the Pick and Hirano bodies in a case of Pick's disease. Acta. Neuropathol. 11, 330–337 (1968).
Goldman, J. E. The association of actin with Hirano bodies. J. Neuropathol. Exp. Neurol. 42, 146–152 (1983).
Galloway, P. G., Perry, G. & Gambetti, P. Hirano body filaments contain actin and actin-associated proteins. J. Neuropathol. Exp. Neurol. 46, 185–199 (1987).
Hirano, A. Hirano bodies and related neuronal inclusions. Neuropathol. Appl. Neurobiol. 20, 3–11 (1994).
Gourlay, C. W., Carpp, L. N., Timpson, P., Winder, S. J. & Ayscough, K. R. A role for the actin cytoskeleton in cell death and aging in yeast. J. Cell Biol. 164, 803–809 (2004).
Ohtsu, M. et al. Inhibition of apoptosis by the actin-regulatory protein gelsolin. EMBO J. 16, 4650–4656 (1997).
Koya, R. C. et al. Gelsolin inhibits apoptosis by blocking mitochondrial membrane potential loss and cytochrome c release. J. Biol. Chem. 275, 15343–15349 (2000).
Chua, B. T. et al. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nature Cell Biol. 5, 1083–1089 (2003).
Wittmann, C. W. et al. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293, 711–714 (2001).
Jackson, G. R. et al. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron 34, 509–519 (2002).
Muqit, M. M. & Feany, M. B. Modelling neurodegenerative diseases in Drosophila: a fruitful approach? Nature Rev. Neurosci. 3, 237–243 (2002).
Nishimura, I., Yang, Y. & Lu, B. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell 116, 671–682 (2004).
Karsten, S. L. et al. A genomic screen for modifiers of tauopathy identifies puromycin-sensitive aminopeptidase as an inhibitor of tau-induced neurodegeneration. Neuron 51, 549–560 (2006).
Andorfer, C. et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 86, 582–590 (2003).
Andorfer, C. et al. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J. Neurosci. 25, 5446–5454 (2005).
Ramsden, M. et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J. Neurosci. 25, 10637–10647 (2005).
Santacruz, K. et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481 (2005).
McGough, A., Pope, B., Chiu, W. & Weeds, A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J. Cell Biol. 138, 771–781 (1997).
Galloway, P. G., Perry, G., Kosik, K. S. & Gambetti, P. Hirano bodies contain tau protein. Brain Res. 403, 337–340 (1987).
Maciver, S. K. & Harrington, C. R. Two actin binding proteins, actin depolymerizing factor and cofilin, are associated with Hirano bodies. Neuroreport 6, 1985–1988 (1995).
Spires, T. L. et al. Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am. J. Pathol. 168, 1598–1607 (2006).
Shulman, J. M. & Feany, M. B. Genetic modifiers of tauopathy in Drosophila. Genetics 165, 1233–1242 (2003).
Khurana, V. et al. TOR-mediated cell-cycle activation causes neurodegeneration in a Drosophila tauopathy model. Curr. Biol. 16, 230–241 (2006).
Wagner, C. R., Mahowald, A. P. & Miller, K. G. One of the two cytoplasmic actin isoforms in Drosophila is essential. Proc. Natl Acad. Sci. USA 99, 8037–8042 (2002).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Gotz, J., Chen, F., van Dorpe, J. & Nitsch, R. M. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Aβ 42 fibrils. Science 293, 1491–1495 (2001).
Lewis, J. et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293, 1487–1491 (2001).
Finelli, A., Kelkar, A., Song, H. J., Yang, H. & Konsolaki, M. A model for studying Alzheimer's Aβ42-induced toxicity in Drosophila melanogaster. Mol. Cell. Neurosci. 26, 365–375 (2004).
Ghosh, S. & Feany, M. B. Comparison of pathways controlling toxicity in the eye and brain in Drosophila models of human neurodegenerative diseases. Hum. Mol. Genet. 13, 2011–2018 (2004).
Warrick, J. M. et al. Expanded polyglutamine protein forms nuclear inclusions and causes neural degeneration in Drosophila. Cell 93, 939–949 (1998).
Harms, C. et al. Neuronal gelsolin prevents apoptosis by enhancing actin depolymerization. Mol. Cell Neurosci. 25, 69–82 (2004).
Zhu, X. et al. Oxidative stress signalling in Alzheimer's disease. Brain Res. 1000, 32–39 (2004).
Rodriguez, O. C. et al. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nature Cell Biol. 5, 599–609 (2003).
Niwa, R., Nagata-Ohashi, K., Takeichi, M., Mizuno, K. & Uemura, T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate Alzheimer's diseaseF/cofilin. Cell 108, 233–246 (2002).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999).
Dias-Santagata, D., Fulga, T. A., Duttaroy, A. & Feany, M. B. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J. Clin. Invest. 117, 236–245 (2007).
Acknowledgements
We are grateful to those who generously sent us stocks and reagents (see Methods). Fly injection services were provided by D. Rennie at the Cutaneous Biology Research Center at Massachusetts General Hospital. We thank R. Stearns for help with the SEM facility. The rTg4510 mice were provided by G. Carlson and K. Hsiao Ashe. This work was supported by National Institutes of Health (NIH) grants AG19790 and AG5134 and a McKnight Foundation grant to M.B.F. T.A.F is the recipient of a postdoctoral fellowship from The John Douglas French Alzheimer's Foundation.
Author information
Authors and Affiliations
Contributions
T.A.F., I.E.-S., V.K. and M.B.F. contributed to study design, data acquisition and manuscript preparation. M.L.S., T.L.S. and B.T.H. contributed critical reagents and assisted with data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3 and S4 (PDF 701 kb)
Rights and permissions
About this article
Cite this article
Fulga, T., Elson-Schwab, I., Khurana, V. et al. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol 9, 139–148 (2007). https://doi.org/10.1038/ncb1528
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1528
This article is cited by
-
Evolving prion-like tau conformers differentially alter postsynaptic proteins in neurons inoculated with distinct isolates of Alzheimer’s disease tau
Cell & Bioscience (2023)
-
Simple model systems reveal conserved mechanisms of Alzheimer’s disease and related tauopathies
Molecular Neurodegeneration (2023)
-
DLK-MAPK Signaling Coupled with DNA Damage Promotes Intrinsic Neurotoxicity Associated with Non-Mutated Tau
Molecular Neurobiology (2023)
-
Prion disease modelled in Drosophila
Cell and Tissue Research (2023)
-
Long COVID could become a widespread post-pandemic disease? A debate on the organs most affected
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)