Abstract
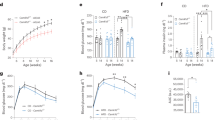
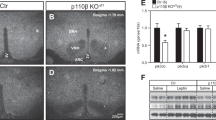
Obesity is an epidemic in Western society, and causes rapidly accelerating rates of type 2 diabetes and cardiovascular disease. The evolutionarily conserved serine/threonine kinase, AMP-activated protein kinase (AMPK), functions as a ‘fuel gauge’ to monitor cellular energy status1. We investigated the potential role of AMPK in the hypothalamus in the regulation of food intake. Here we report that AMPK activity is inhibited in arcuate and paraventricular hypothalamus (PVH) by the anorexigenic hormone leptin, and in multiple hypothalamic regions by insulin, high glucose and refeeding. A melanocortin receptor agonist, a potent anorexigen2, decreases AMPK activity in PVH, whereas agouti-related protein, an orexigen2, increases AMPK activity. Melanocortin receptor signalling is required for leptin and refeeding effects on AMPK in PVH. Dominant negative AMPK expression in the hypothalamus is sufficient to reduce food intake and body weight, whereas constitutively active AMPK increases both. Alterations of hypothalamic AMPK activity augment changes in arcuate neuropeptide expression induced by fasting and feeding. Furthermore, inhibition of hypothalamic AMPK is necessary for leptin's effects on food intake and body weight, as constitutively active AMPK blocks these effects. Thus, hypothalamic AMPK plays a critical role in hormonal and nutrient-derived anorexigenic and orexigenic signals and in energy balance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hardie, D. G., Scott, J. W., Pan, D. A. & Hudson, E. R. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 546, 113–120 (2003)
Schwartz, M. W. et al. Central nervous system control of food intake. Nature 404, 661–671 (2000)
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998)
Brüning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000)
Levin, B. E. Glucosensing neurons do more than just sense glucose. Int. J. Obes. Relat. Metab. Disord. Suppl. 5, S68–S72 (2001)
Obici, S. et al. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 5, 271–275 (2002)
Hawley, S. A. et al. Complexes between the LKB1 tumor suppressor, STRADα/β and MO25α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2(28), 1–16 (2003)
Turnley, A. M. et al. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J. Neurochem. 72, 1707–1716 (1999)
Culmsee, C., Monnig, J., Kemp, B. E. & Mattson, M. P. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J. Mol. Neurosci. 17, 45–58 (2001)
Elmquist, J. K., Elias, C. F. & Saper, C. B. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22, 221–232 (1999)
Obici, S., Zhang, B. B., Karkanias, G. & Rossetti, L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nature Med. 8, 1376–1382 (2002)
Ollmann, M. M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1977)
Woods, A. et al. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell. Biol. 20, 6704–6711 (2000)
Viollet, B. et al. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J. Clin. Invest. 111, 91–98 (2003)
Andersson, U. et al. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. published online 23 January 2004 (doi:10.1074/jbc.C300557200)
Bates, S. H. et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421, 856–859 (2003)
Niswender, K. D. et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413, 794–795 (2001)
Zhao, A.-Z. et al. A phosphatidylinositol 3-kinase-phosphodiesterase 3B-cyclic AMP in hypothalamic action of leptin on feeding. Nature Neurosci. 5, 727–728 (2002)
Obici, S. et al. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nature Med. 9, 756–761 (2003)
Cowley, M. A. et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163 (1999)
Light, P. E., Wallace, C. H. R. & Dyck, J. R. B. Constitutively active adenosine monophosphate-activated protein kinase regulates voltage-gated sodium channels. Circulation 107, 1962–1965 (2003)
Hallows, K. R. et al. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J. Clin. Invest. 12, 1711–1721 (2000)
da Silva Xavier, G. et al. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem. J. 371, 761–774 (2003)
Spanswick, D. et al. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390, 521–525 (1997)
Spanswick, D. et al. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nature Neurosci. 3, 757–758 (2000)
Loftus, T. M. et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science 288, 2379–2381 (2000)
Hu, Z., Cha, S. H., Chohnan, S. & Lane, D. Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc. Natl Acad. Sci. USA 100, 12624–12629 (2003)
Ruderman, N. B., Saha, A. K., Vavvas, E. & Witters, L. A. Malonyl-CoA, fuel sensing, and insulin resistance. Am. J. Physiol. 276, E1–E18 (1999)
Minokoshi, Y. et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415, 339–343 (2002)
Woods, S. et al. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 397, 347–351 (1996)
Hayashi, T. et al. Metabolic stress and altered glucose transport. Activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes 49, 527–531 (2000)
Acknowledgements
We thank D. Carling for reagents and advice, J. K. Elmquist and B. B. Lowell for discussions and providing MC4R-KO mice, and C. J. Aschkenasi, C.-Y. Zhang, O. Boss, J. Yu and N. Balthasar for MC4R-KO mice. This work was supported by NIH grants (B.B.K. and M.J.B.), an EASD-ADA and Bettencourt-Schueller Foundation Fellowship (T.A.), AMPDIAMET (P.F.) and the American Diabetes Association (B.B.K. and Y.B.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Supplementary Figure 1
mRNA levels of neuropeptides in dissected hypothalamic samples. (PDF 65 kb)
Supplementary Figure 2
Protein expression of α2 subunit of AMPK and STAT3 in ARH and VMH/DMH. (PDF 73 kb)
Rights and permissions
About this article
Cite this article
Minokoshi, Y., Alquier, T., Furukawa, N. et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569–574 (2004). https://doi.org/10.1038/nature02440
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature02440
This article is cited by
-
Nutritional strategies cause memory damage and alter biochemical parameters without causing neuroinflammation
Metabolic Brain Disease (2024)
-
Effects of aerobic training and vitamin D supplementation on glycemic indices and adipose tissue gene expression in type 2 diabetic rats
Scientific Reports (2023)
-
A protein kinase C α and β inhibitor blunts hyperphagia to halt renal function decline and reduces adiposity in a rat model of obesity-driven type 2 diabetes
Scientific Reports (2023)
-
CPT1A in AgRP neurons is required for sex-dependent regulation of feeding and thirst
Biology of Sex Differences (2023)
-
eQTL mapping in fetal-like pancreatic progenitor cells reveals early developmental insights into diabetes risk
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.