Key Points
-
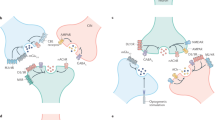
There are two broad classes of ATP receptor in the nervous system: P2X and P2Y receptors. P2X receptors, a group of cation-selective ion channels, are the focus of this review. There are seven different P2X subunits, each of which has two transmembrane domains inserted in the plasma membrane such that both the amino and the carboxyl termini are located intracellularly.
-
The ATP-binding site of P2X receptors seems to be located near the transmembrane domains and is thought to involve several lysine residues. The second transmembrane domain of P2X subunits forms the channel pore, which is permeable to different cations including calcium. Although their exact subunit stochiometry is controversial, most of the evidence supports the idea that P2X receptors assemble as trimers.
-
P2X receptors are ubiquitously expressed in the nervous system. Indeed, they are present both pre- and postsynaptically. Activation of presynaptic P2X receptors can modulate release of several neurotransmitters at a variety of synapses. Postsynaptically, ATP can elicit excitatory synaptic currents in some cells, but they seem to contribute to a small part of the total excitatory drive.
-
P2X receptors have been implicated in processing pain information. ATP released from damaged tissue can act directly on P2X receptors present in the primary sensory afferent. In turn, the terminals of sensory neurons release ATP onto dorsal horn cells. In addition, presynaptic receptors on the terminals of the sensory neuron facilitate the release of glutamate onto dorsal horn cells, and presynaptic P2X receptors on inhibitory nerve terminals also facilitate the release of GABA and glycine. The balance between inhibitory and excitatory effects contributes to the spinal processing of painful information.
Abstract
ATP is found in every cell, where it is a major source of energy. But in the nervous system, ATP also has additional actions, which include its role in fast synaptic transmission and modulation. Here I discuss the 'fast' actions of ATP at synapses, the properties of the receptors that are activated by ATP and the physiology of ATP signalling, with emphasis on its role in pain processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Holton, F. A. & Holton, P. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. J. Physiol. (Lond.) 126, 124–140 ( 1954).
Holton, P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J. Physiol. (Lond.) 145, 494– 504 (1959).
Burnstock, G. Neural nomenclature. Nature 229, 282– 283 (1971).References 1 – 3 are early papers that focused attention on ATP release from neurons.
North, R. A. & Barnard, E. A. Nucleotide receptors. Curr. Opin. Neurobiol. 7, 346–357 (1997).
Zhou, X. & Galligan, J. J. P2X purinoceptors in cultured myenteric neurons of guinea-pig small intestine. J. Physiol. (Lond.) 496, 719–729 ( 1996).
Robertson, S. J. & Edwards, F. A. ATP and glutamate are released from separate neurons in the rat medial habenula nucleus: frequency dependence and adenosine-mediated inhibition of release. J. Physiol. (Lond.) 508, 691–701 ( 1998).
Stevens, B. & Fields, R. D. Response of Schwann cells to action potentials in development. Science 287, 2267–2271 (2000).
Cotrina, M. L., Lin, J. H., Lopez-Garcia, J. C., Naus, C. C. & Nedergaard, M. ATP-mediated glia signalling . J. Neurosci. 20, 2835– 2844 (2000).
Guthrie, P. B. et al. ATP released from astrocytes mediates glial calcium waves . J. Neurosci. 19, 520– 528 (1999).
Powell, A. D., Teschemacher, A. G. & Seward, E. P. P2Y purinoceptors inhibit exocytosis in adrenal chromaffin cells via modulation of voltage-operated calcium channels. J. Neurosci. 20, 606–616 ( 2000).
Currie, K. P. & Fox, A. P. ATP serves as a negative feedback inhibitor of voltage-gated Ca2+ channel currents in cultured bovine adrenal chromaffin cells. Neuron 16, 1027–1036 (1996).
Hollins, B. & Ikeda, S. R. Heterologous Expression of a P2X-purinoceptor in rat chromaffin cells detects vesicular ATP release. J. Neurophysiol. 78, 3069–3076 ( 1997).
Burnashev, N. Calcium permeability of ligand-gated channels. Cell Calcium 24, 325–332 (1998).
Berridge, M. J. Neuronal calcium signaling. Neuron 21, 13 –26 (1998).
Zimmermann, H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 362, 299–309 (2000).
Torres, G., Egan, T. & Voigt, M. Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J. Biol. Chem. 274, 6653–6659 (1999).
Unwin, N. Acetylcholine receptor channel imaged in the open state. Nature 373, 37–43 ( 1995).
Doyle, D. A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
Chang, G., Spencer, R. H., Lee, A. T., Barclay, M. T. & Rees, D. C. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282, 2220–2226 ( 1998).
North, R. A. & Surprenant, A. Pharmacology of cloned P2X receptors . Annu. Rev. Pharmacol. Toxicol 40, 563– 580 (2000).
Newbolt, A. et al. Membrane topology of an ATP-gated ion channel (P2X receptor) . J. Biol. Chem. 273, 15177– 15182 (1998).
Torres, G. E., Egan, T. M. & Voigt, M. M. N-Linked glycosylation is essential for the functional expression of the recombinant P2X2 receptor. Biochemistry 37, 14845–14851 (1998).
Boue-Grabot, E., Archambault, V. & Seguela, P. A Protein kinase C Site highly conserved in P2X subunits controls the desensitization kinetics of P2X2 ATP-gated channels . J. Biol. Chem. 275, 10190– 10195 (2000).
Zhou, Z., Monsma, L. R. & Hume, R. I. Identification of a site that modifies desensitization of P2X2 receptors. Biochem. Biophys. Res. Commun. 252, 541–546 (1998).
Kim, M., Yoo, O. J. & Choe, S. Molecular assembly of the extracellular domain of P2X 2, an ATP-gated ion channel. Biochem. Biophys. Res. Commun. 240, 618–622 ( 1997).The extracellular domain of P2X 2 subunits was shown to assemble as tetramer.
Clarke, C. E., Benham, C. D., Bridges, A., George, A. R. & Meadows, H. J. Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity . J. Physiol. (Lond.) 523, 697– 703 (2000).
Nakazawa, K. & Ohno, Y. Neighboring glycine residues are essential for P2X2 receptor/channel function. Eur. J. Pharmacol. 370, R5–R6 ( 1999).
Rettinger, J., Aschrafi, A. & Schmalzing, G. Roles of individual-glycans for ATP potency and expression of the rat P2X1 receptor. J. Biol. Chem. 275, 33542–33547 (2000).
Ennion, S., Hagan, S. & Evans, R. J. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J. Biol. Chem. 275, 29361–29367 (2000).
Jiang, L.-H., Rassendren, F., Surprenant, A. & North, R. A. Identification of amino acid residues contributing to the ATP binding site of a P2X receptor. J. Biol. Chem. 275, 34190 –34196 (2000).References 29 and 30 identify residues that affect ATP potency and that probably contribute to the ATP-binding site.
Buell, G., Lewis, C., Collo, G., North, R. A. & Surprenant, A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 15, 55– 62 (1996).
Evans, R. J. et al. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J. Physiol. (Lond.) 497, 413–422 (1996).
Koshimizu, T. et al. Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol. Pharmacol. 58, 936–945 (2000).
Edwards, F. A., Robertson, S. J. & Gibb, A. J. Properties of ATP receptor-mediated synaptic transmission in the rat medial habenula. Neuropharmacology 36, 1253–1268 (1997).
Rogers, M. & Dani, J. A. Comparison of quantitative calcium flux through NMDA, ATP, and ACh receptor channels. Biophys. J. 68, 501–506 ( 1995).
Rogers, M., Colquhoun, L. M., Patrick, J. W. & Dani, J. A. Calcium flux through predominantly independent purinergic ATP and nicotinic acetylcholine receptors. J. Neurophysiol. 77, 1407–1417 (1997).
Rassendren, F., Buell, G., Newbolt, A., North, R. A. & Surprenant, A. Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO J. 16, 3446–3454 (1997).
Egan, T. M., Haines, W. R. & Voigt, M. M. A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J. Neurosci. 18, 2350– 2359 (1998).References 37 and 38 show that transmembrane domain 2 lines the pore of P2X 2 subunits.
Surprenant, A., Rassendren, F., Kawashima, E., North, R. A. & Buell, G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272, 735–738 (1996). Cloning and expression of the P2X 7 subunit, and the demonstration of its ability to change its permeability.
Khakh, B. S., Proctor, W. R., Dunwiddie, T. V., Labarca, C. & Lester, H. A. Allosteric control of gating and kinetics at P2X4 receptor channels. J. Neurosci. 19, 7289–7299 (1999).
Khakh, B., Bao, X., Labarca, C. & Lester, H. Neuronal P2X receptor-transmitter-gated cation channels change their ion selectivity in seconds. Nature Neurosci. 2, 322–330 ( 1999).
Virginio, C., MacKenzie, A., Rassendren, F. A., North, R. A. & Surprenant, A. Pore dilation of neuronal P2X receptor channels. Nature Neurosci. 2, 315 –321 (1999).References 41 and 42 show that neuronal P2X receptors change their permeability, and that transmembrane domain 2 contributes to this phenomenon.
Virginio, C., MacKenzie, A., North, R. A. & Surprenant, A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J. Physiol. (Lond.) 519, 335–346 (1999).
Khakh, B. S. & Lester, H. A. Dynamic selectivity filters in ion channels. Neuron 23, 653– 658 (1999).
Surprenant, A., Schneider, D. A., Wilson, H. L., Galligan, J. J. & North, R. A. Functional properties of heteromeric P2X1/5 receptors expressed in HEK cells and excitatory junction potentials in guinea-pig submucosal arterioles. J. Auton. Nerv. Syst. 81, 249–263 (2000).
Rassendren, F. et al. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 272, 5482–5786 (1997).
Nakazawa, K., Fujimori, K., Takanaka, A. & Inoue, K. Comparison of adenosine triphosphate- and nicotine-activated inward currents in rat phaeochromocytoma cells. J. Physiol. (Lond.) 434, 647–660 (1991).
Cohen, B. N., Labarca, C., Davidson, N. & Lester, H. A. Mutations in M2 alter the selectivity of the mouse nicotinic acetylcholine receptor for organic and alkali metal cations. J. Gen. Physiol. 100, 373–400 ( 1992).
Brandle, U. et al. Desensitization of the P2X2 receptor controlled by alternative splicing. FEBS Lett. 404, 294–298 (1997).
Simon, J. et al. Localization and functional expression of splice variants of the P2X2 receptor. Mol. Pharmacol. 52, 237–248 (1997).
Smith, F. M., Humphrey, P. P. A. & Murrell-Lagnado, R. D. Identification of amino acids within the P2X2 receptor C-terminus that regulate desensitization. J. Physiol. (Lond.) 520, 91–99 (1999).
Werner, P., Seward, E. P., Buell, G. N. & North, R. A. Domains of P2X receptors involved in desensitization. Proc. Natl Acad. Sci. USA 93, 15485– 15490 (1996).
Li, G. H. et al. The distribution of P2X receptor clusters on individual neurons in sympathetic ganglia and their redistribution on agonist activation. J. Biol. Chem. 275, 29107–29112 (2000).
Nicke, A. et al. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 17, 3016–3028 ( 1998).
Torres, G. E., Egan, T. M. & Voigt, M. M. Identification of a domain involved in ATP-gated ionotropic receptor subunit assembly. J. Biol. Chem. 274, 22359–22365 (1999).
Stoop, R. et al. Contribution of individual subunits to the multimeric P2X 2 receptor: estimates based on methanethiosulfonate block at T336C. Mol. Pharmacol. 56, 973–981 (1999).References 54 and 56 show that P2X receptors might be trimers.
Ding, S. & Sachs, F. Single channel properties of P2X 2 purinoceptors. J. Gen. Physiol. 113, 695–720 (1999).The first detailed study of the single-channel behaviour of P2X 2 receptors.
Zhou, Z. & Hume, R. I. Two mechanisms for inward rectification of current flow through the purinoceptor P2X2 class of ATP-gated channels. J. Physiol. (Lond.) 507, 353– 364 (1998).
Ding, S. & Sachs, F. Ion permeation and block of P2X 2 purinoceptors: single channel recordings. J. Membr. Biol. 172, 215–223 ( 1999).
Hackos, D. H. & Korenbrot, J. I. Divalent cation selectivity is a function of gating in native and recombinant cyclic-nucleotide-gated ion channels from retinal photoreceptors. J. Gen. Physiol. 113, 799–817 (1999).
Mulryan, K. et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature 403, 86–89 (2000).The first knockout of a P2X subunit.
Rubio, M. E. & Soto, F. Distinct localisation of P2X receptors at excitatory postsynaptic specializations. J. Neurosci. 21, 641–653 (2001). Detailed localization studies of neuronal P2X receptors in dendrites shows a pattern different from that of glutamate receptors.
Collo, G. et al. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels . J. Neurosci. 16, 2495– 2507 (1996).
Atkinson, L., Batten, T. F. & Deuchars, J. P2X2 receptor immunoreactivity in the dorsal vagal complex and area postrema of the rat. Neuroscience 99, 683–696 (2000).
Krishtal, O. A., Marchenko, S. M. & Pidoplichko, V. I. Receptor for ATP in the membrane of mammalian sensory neurons. Neurosci. Lett. 31, 41– 45 (1983).
Jahr, C. E. & Jessell, T. M. ATP excites a subpopulation of rat dorsal horn neurons. Nature 304, 730 –733 (1983).
Grubb, B. & Evans, R. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur. J. Neurosci. 11, 149–154 (1999).
Lewis, C. et al. Co-expression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 377, 432–435 ( 1995).
Virginio, C., North, R. A. & Surprenant, A. Calcium permeability and block at homomeric and heteromeric P2X2 and P2X3 receptors, and P2X receptors in rat nodose neurons. J. Physiol. (Lond.) 510, 27– 35 (1998).
Virginio, C., Robertson, G., Surprenant, A. & North, R. A. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors . Mol. Pharmacol. 53, 969– 973 (1998).
Vulchanova, L. et al. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl Acad. Sci. USA 93, 8063–8067 (1996).
Vulchanova, L. et al. Immunohistochemical study of the P2X2 and P2X 3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology 36, 1229– 1242 (1997).
Chen, C. C. et al. A P2X purinoceptor expressed by a subset of sensory neurons . Nature 377, 428–431 (1995).
Le, K. T., Babinski, K. & Séguéla, P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J. Neurosci. 18, 7152–7159 (1998).
Patel, M., Khakh, B. S. & Henderson, G. Characterisation of P2X receptors in trigeminal mesecephalic nucleus. Neuropharmacology 40, 96– 105 (2001).
Cook, S. P., Vulchanova, L., Hargreaves, K. M., Elde, R. & McCleskey, E. W. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature 387, 505–508 (1997).The first detailed study of P2X receptors on identified pain-sensing neurons.
Khakh, B. S., Humphrey, P. P. & Henderson, G. ATP-gated cation channels (P2X purinoceptors) in trigeminal mesencephalic nucleus neurons of the rat. J. Physiol. (Lond.) 498, 709–715 (1997).
Torres, G. E., Haines, W. R., Egan, T. M. & Voigt, M. M. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol. Pharmacol. 54, 989–993 (1998).
Khakh, B. S. & Henderson, G. Modulation of fast synaptic transmission by presynaptic ligand-gated cation channels. J. Auton. Nerv. Syst. 81, 110–121 ( 2000).
Le, K. T. et al. Sensory presynaptic and widespread somatodendritic immunolocalization of central ionotropic P2X ATP receptors. Neuroscience 83, 177–190 (1998).
Sun, X. P. & Stanley, E. F. An ATP-activated, ligand-gated ion channel on a cholinergic presynaptic nerve terminal. Proc. Natl Acad. Sci. USA 93, 1859–1863 (1996).
Fu, W. M. & Poo, M.-M. ATP potentiates spontaneous transmitter release at developing neuromuscular synapses. Neuron 6, 837–843 (1991).
Gu, J. G. & MacDermott, A. B. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 389, 749–753 ( 1997).
Hugel, S. & Schlichter, R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J. Neurosci. 20, 2121– 2130 (2000).
Khakh, B. S. & Henderson, G. ATP receptor-mediated enhancement of fast excitatory neurotransmitter release in the brain. Mol. Pharmacol. 54, 372–378 ( 1998).References 83 – 85 show that presynaptic P2X receptors regulate fast synaptic transmission.
Boehm, S. ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors . J. Neurosci. 19, 737– 746 (1999).
Fu, W. M. & Liu, J. J. Regulation of acetylcholine release be presynaptic nicotinic receptors at developing neuromuscular synapses. Mol. Pharmacol. 51, 390–398 (1997).
Burnstock, G. Purinergic nerves. Pharmacol. Rev. 24, 509 –581 (1972).
Evans, R. J., Derkach, V. & Surprenant, A. ATP mediates fast synaptic transmission in mammalian neurons. Nature 357, 503– 505 (1992).
Silinsky, E. M., Gerzanich, V. & Vanner, S. M. ATP mediates excitatory synaptic transmission in mammalian neurons. Br. J. Pharmacol. 106, 762–763 (1992).
Silinsky, E. M. & Gerzanich, V. On the excitatory effects of ATP and its role as a neurotransmitter in coeliac neurons of the guinea-pig. J. Physiol. (Lond.) 464, 197 –212 (1993).
Galligan, J. J. & Bertrand, P. P. ATP mediates fast synaptic potentials in enteric neurons. J. Neurosci. 14, 7563–7571 (1994).
Edwards, F. A., Gibb, A. J. & Colquhoun, D. ATP receptor-mediated synaptic currents in the central nervous system. Nature 359, 144– 147 (1992).
Robertson, S. J., Burnashev, N. & Edwards, F. A. Ca2+ permeability and kinetics of glutamate receptors in rat medial habenula neurons: implications for purinergic transmission in this nucleus. J. Physiol. (Lond.) 518 , 539–549 (1999).
Nieber, K., Poelchen, W. & Illes, P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurons of the rat. Br. J. Pharmacol. 122, 423–430 (1997).
Bardoni, R., Goldstein, P. A., Lee, C. J., Gu, J. G. & MacDermott, A. B. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J. Neurosci. 17, 5297–5304 (1997).
Jo, Y.-H. & Schlichter, R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nature Neurosci. 2 , 241–245 (1999).
Pankratov, Y., Castro, E., Miras-Portugal, M. T. & Krishtal, O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur. J. Neurosci. 10, 3898–3902 (1998).
Gribble, F. M. et al. A novel method for measurement of sub-membrane ATP concentration . J. Biol. Chem. 275, 30046– 30049 (2000).
Bleehen, T. & Keele, C. A. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain 3, 367–377 ( 1977).
Coutts, A. A., Jorizzo, J. L., Eady, R. A., Greaves, M. W. & Burnstock, G. Adenosine triphosphate-evoked vascular changes in human skin: mechanism of action. Eur. J. Pharmacol. 76, 391–401 ( 1981).
Hamilton, S. G., Warburton, J., Bhattacharjee, A., Ward, J. & McMahon, S. B. ATP in human skin elicits a dose-related pain response which is potentiated under conditions of hyperalgesia. Brain 123, 1238–1246 ( 2000).
Bland-Ward, P. A. & Humphrey, P. P. Acute nociception mediated by hindpaw P2X receptor activation in the rat. Br. J. Pharmacol. 122, 365–371 (1997).
Dowd, E., McQueen, D. S., Chessell, I. P. & Humphrey, P. P. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joInt Br. J. Pharmacol. 125, 341–346 (1998).
Kirkup, A. J., Booth, C. E., Chessell, I. P., Humphrey, P. P. & Grundy, D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J. Physiol. (Lond.) 520, 551–563 (1999).
Sawynok, J. & Reid, A. Peripheral adenosine 5′-triphosphate enhances nociception in the formalin test via activation of a purinergic P2X receptor. Eur. J. Pharmacol. 330, 115– 121 (1997).
McQueen, D. S. et al. Activation of P2X receptors for adenosine triphosphate evokes cardiorespiratory reflexes in anaesthetized rats. J. Physiol. (Lond.) 507, 843–855 ( 1998).
Cook, S. P. & McCleskey, E. W. Rapid activation of nociceptor P2X receptors by damage to nearby cells. Soc. Neurosci. Abstr. 39, 17 (2000).
Hamilton, S. G., Wade, A. & McMahon, S. B. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br. J. Pharmacol. 126, 326–332 (1999).
Krishtal, O. A., Marchenko, S. M. & Obukhov, A. G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience 27, 995–1000 (1988).
Krishtal, O. A., Marchenko, S. M., Obukhov, A. G. & Volkova, T. M. Receptors for ATP in rat sensory neurons: the structure-function relationship for ligands. Br. J. Pharmacol. 95, 1057– 1062 (1988).
Khakh, B. S., Humphrey, P. P. & Surprenant, A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurons. J. Physiol. (Lond.) 484, 385–395 (1995).
Robertson, S. J., Rae, M. G., Rowan, E. G. & Kennedy, C. Characterisation of a P2X-purinoceptor in cultured neurons of the rat dorsal root ganglia. Br. J. Pharmacol. 118, 951–956 (1996).
Rae, M. G., Rowan, E. G. & Kennedy, C. Pharmacological properties of P2X3-receptors present in neurons of the rat dorsal root ganglia. Br. J. Pharmacol. 124, 176–180 ( 1998).
Thomas, S., Virginio, C., North, R. A. & Surprenant, A. The antagonist trinitrophenyl-ATP reveals coexistence of distinct P2X receptor channels in rat nodose neurons. J. Physiol. (Lond.) 509, 411–417 (1998).
Cockayne, D. A. et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature 407, 1011–1015 (2000).
Souslova, V. et al. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature 407, 1015–1017 (2000). References 116 and 117 report on pain-related aspects of P2X 3 -knockout mice.
Rhee, J. S., Wang, Z. M., Nabekura, J., Inoue, K. & Akaike, N. ATP facilitates spontaneous glycinergic IPSC frequency at dissociated rat dorsal horn interneuron synapses. J. Physiol. (Lond.) 524, 471–483 (2000).
Tsuda, M., Ueno, S. & Inoue, K. Evidence for the involvement of spinal endogenous ATP and P2X receptors in nociceptive responses caused by formalin and capsaicin in mice. Br. J. Pharmacol. 128, 1497–1504 (1999).
Stanfa, L. C., Kontinen, V. K. & Dickenson, A. H. Effects of spinally administered P2X receptor agonists and antagonists on the responses of dorsal horn neurons recorded in normal, carrageenan-inflamed and neuropathic rats. Br. J. Pharmacol. 129, 351–359 (2000).
Green, T., Heinemann, S. F. & Gusella, J. F. Molecular Neurobiology and genetics: investigation of neural function and dysfunction. Neuron 20, 427–444 (1998).
Ranganathan, R., Cannon, S. C. & Horvitz, H. R. MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 408, 470–475 (2000).
Cully, D. F. et al. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 371, 707–711 (1994).
Chen, G. Q., Cui, C., Mayer, M. L. & Gouaux, E. Functional characterization of a potassium-selective prokaryotic glutamate receptor. Nature 402, 817–821 ( 1999).
Brake, A. J., Wagenbach, M. J. & Julius, D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 371, 519–523 (1994).
Davies, P. A. et al. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397, 359– 363 (1999).
Dingledine, R., Borges, K., Bowie, D. & Traynelis, S. F. The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61 (1999).
Lukas, R. J. et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits . Pharmacol. Rev. 51, 397– 401 (1999).
North, R. A. Families of ion channels with two hydrophobic segments. Curr. Opin. Cell Biol. 8, 474–483 ( 1996).
Valera, S. et al. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature 371, 516– 519 (1994).
Waldmann, R., Champigny, G., Bassilana, F., Heurteaux, C. & Lazdunski, M. A proton-gated cation channel involved in acid-sensing. Nature 386, 173 –177 (1997).
Lingueglia, E., Champigny, G., Lazdunski, M. & Barbry, P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel . Nature 378, 730–733 (1995).
Burnstock, G. The past, present and future of purine nucleotides as signalling molecules . Neuropharmacology 36, 1127– 1139 (1997).
Nakazawa, K. ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J. Neurosci. 14, 740–750 (1994).
Searl, T. J., Redman, R. S. & Silinsky, E. M. Mutual occlusion of P2X ATP receptors and nicotinic receptors on sympathetic neurons of the guinea-pig. J. Physiol. (Lond.) 510, 783–791 ( 1998).
Zhou, X. & Galligan, J. J. Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J. Physiol. (Lond.) 513, 685 –697 (1998).
Barajas-Lopez, C., Espinosa-Luna, R. & Zhu, Y. Functional interactions between nicotinic and P2X channels in short-term cultures of guinea-pig submucosal neurons. J. Physiol. (Lond.) 513, 671–683 ( 1998).
Khakh, B. S., Zhou, X., Sydes, J., Galligan, J. J. & Lester, H. A. State-dependent cross-inhibition between transmitter-gated cation channels. Nature 406, 405– 410 (2000).
Lutz, P. L. & Kabler, S. Release of adenosine and ATP in the brain of the freshwater turtle (Trachemys scripta) during long-term anoxia. Brain Res. 769, 281– 286 (1997).
Bueno, L. & Fioramonti, J. Effects of inflammatory mediators on gut sensitivity. Can. J. Gastroenterol. 13, 42A–46A (1999).
Taleb, O. & Betz, H. Expression of the human glycine receptor α1 subunit in Xenopus oocytes: apparent affinities of agonists increase at high receptor density. EMBO J. 13, 1318 –1324 (1994).
Honore, E. et al. Different types of K+ channel current are generated by different levels of a single mRNA. EMBO J. 11, 2465–2471 (1992).
Duke, T. A. & Bray, D. Heightened sensitivity of a lattice of membrane receptors. Proc. Natl Acad. Sci. USA 96 , 10104–10108 (1999).
Liu, F. et al. Direct protein–protein coupling enables cross-talk between dopamine D5 and γ-aminobutyric acid A receptors. Nature 403, 274–280 (2000).
Dalva, M. B. et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103, 957– 969 (2000).
Acknowledgements
While at Caltech, B.S.K. was supported by a Wellcome Trust (UK) International Prize Travelling Research Fellowship and Roche Bioscience (Palo Alto). Many thanks to Cesar Labarca for comments on the paper and to Henry A. Lester for encouragement.
Author information
Authors and Affiliations
Supplementary information
Related links
Glossary
- AUERBACHS PLEXUS
-
A collection of nerves and ganglia located between the circular and longitudinal muscles in the small intestine. It is also known as the myenteric plexus.
- CHROMAFFIN CELLS
-
Cells of the adrenal gland that store and secrete catecholamines. They are termed 'chromaffin' because of the ability of chromium salts to stain them.
- ECTOATPASES
-
A general term for the extracellular enzymes that hydrolyse ATP.
- LEADER SEQUENCE
-
A stretch of hydrophobic residues at the amino-terminal end of some proteins, which directs them to the plasma membrane or to the membrane of specific cellular organelles.
- P REGION
-
A conserved structural motif found in all K+-selective ion channels, which constitutes part of the channel pore.
- POTENCY
-
A measure of the change in response as a function of ligand concentration. Agonist potency is quantified as the concentration of ligand that produces half the maximal effect (EC50). The classic pharmacological definition of potency thus includes components of affinity and efficacy. In such schemes, efficacy is simply the ability of a drug to evoke a response once bound. Potency is related to affinity, but potency and affinity are different measures of drug action.
- ALANINE SCANNING MUTAGENESIS
-
This method is used to obtain a preliminary profile of the functionally important regions of a protein. It is based on the systematic replacement of amino acids into alanine and the subsequent analysis of the effect of the mutation on the function of the protein.
- SCHIFF BASE
-
A reversible bond formed by the condensation of carbonyl and amino groups.
- SUBSTITUTED CYSTEINE ACCESSIBILITY MUTAGENESIS
-
A method used to identify the residues that are likely to line the pore of a channel. It is based on the systematic replacement of native amino acids by cysteines to then test the ability of hydrophilic molecules to react with the added cysteines. If a cysteine is accessible to the hydrophilic reagent, then channel permeability will be affected.
- SYNAPTOSOME
-
The presynaptic terminal isolated after subcellular fractionation. This structure retains the anatomical integrity of the terminal and can take up, store and release neurotransmitters.
- MINIATURE EPSCs
-
Excitatory synaptic currents observed in the absence of presynaptic action potentials, thought to correspond to the response elicited by a single vesicle of transmitter.
Rights and permissions
About this article
Cite this article
Khakh, B. Molecular physiology of p2x receptors and atp signalling at synapses . Nat Rev Neurosci 2, 165–174 (2001). https://doi.org/10.1038/35058521
Issue Date:
DOI: https://doi.org/10.1038/35058521
This article is cited by
-
Embryos assist morphogenesis of others through calcium and ATP signaling mechanisms in collective teratogen resistance
Nature Communications (2024)
-
Migraine signaling pathways: purine metabolites that regulate migraine and predispose migraineurs to headache
Molecular and Cellular Biochemistry (2023)
-
P2X7 receptors and pannexin1 hemichannels shape presynaptic transmission
Purinergic Signalling (2023)
-
Oligodendrocyte lineage cells and depression
Molecular Psychiatry (2021)
-
Glial ATP and Large Pore Channels Modulate Synaptic Strength in Response to Chronic Inactivity
Molecular Neurobiology (2020)