Abstract
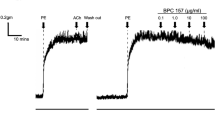
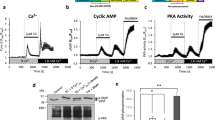
Nitric oxide (NO) produced by the endothelial NO synthase (eNOS) is a fundamental determinant of cardiovascular homesotasis: it regulates systemic blood pressure, vascular remodelling and angiogenesis1,2,3. Physiologically, the most important stimulus for the continuous formation of NO is the viscous drag (shear stress) generated by the streaming blood on the endothelial layer4,5,6,7,8. Although shear-stress-mediated phosphorylation of eNOS is thought to regulate enzyme activity9,10, the mechanism of activation of eNOS is not yet known. Here we demonstrate that the serine/threonine protein kinase Akt/PKB11,12,13 mediates the activation of eNOS, leading to increased NO production. Inhibition of the phosphatidylinositol-3-OH kinase/Akt pathway or mutation of the Akt site on eNOS protein (at serine 1177) attenuates the serine phosphorylation and prevents the activation of eNOS. Mimicking the phosphorylation of Ser 1177 directly enhances enzyme activity and alters the sensitivity of the enzyme to Ca2+, rendering its activity maximal at sub-physiological concentrations of Ca2+. Thus, phosphorylation of eNOS by Akt represents a novel Ca2+-independent regulatory mechanism for activation of eNOS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Moncada, S. & Higgs, A. The L-arginine–nitric oxide pathway. N. Engl. J. Med. 329, 2002–2012 (1993).
Rudic, R. D. et al. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Invest. 101, 731–736 (1998).
Murohara, T. et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Invest. 101, 2567–2578 (1998).
Rubanyi, G. M., Romero, J. C. & Vanhoutte, P. M. Flow-induced release of endothelium-derived relaxing factor. Am. J. Physiol. 250, H1145–H1149 (1986).
Kuchan, M. J. & Frangos, J. A. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am. J. Physiol. 266, C628–C636 (1994).
Sessa, W. C., Pritchard, K., Seyedi, N., Wang, J. & Hintze, T. H. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ. Res. 74, 349–353 (1994).
Davies, P. F. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75, 519–560 (1995).
Busse, R. & Fleming, I. Pulsatile stretch and shear: physiological stimuli determining the production of endothelial-derived relaxing factor. J. Vasc. Res. 35, 73–84 (1998).
Corson, M. A. et al. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ. Res. 79, 984–991 (1996).
Fleming, I., Bauersachs, J., Fissthaler, B. & Busse, R. Ca2+-independent activation of the endothelial nitric oxide synthase in response to tryosine phosphatase inhibitors and fluid shear stress. Circ. Res. 79, 984–991 (1996).
Franke, T. F. et al. The protein kinase encoded by the Akt proto-oncogen is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81, 727–736 (1995).
Burgering, B. M. T. & Coffer, P. J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376, 599–602 (1995).
Downward, J. Lipid-regulating kinases: some common themes at last. Science 279, 673–674 (1998).
Zeiher, A. M. Endothelial vasodilator dysfunction: pathogenetic link to myocardial ischaemia or epiphenomenon? Lancet 348, S10–S12 (1996).
Moroi, M. et al. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular responses to injury in mice. J. Clin. Invest. 101, 1225–1232 (1998).
Bredt, D. S. et al. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature 351, 714–718 (1991).
Busse, R. & Mülsch, A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 265, 133–136 (1990).
Ayajiki, K., Kindermann, M., Hecker, M., Fleming, I. & Busse, R. Intracellular pH and tyrosine phosphorylation but not calcium determine shear stress-induced nitric oxide production in native endothelial cells. Circ. Res. 78, 750–758 (1996).
Dimmeler, S., Assmus, B., Hermann, C., Haendeler, J. & Zeiher, A. M. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ. Res. 83, 334–342 (1998).
Alessi, D. R. et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 (1996).
van der Zee, R. et al. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation 95, 1030–1037 (1997).
Ziche, M. et al. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J. Clin. Invest. 99, 2625–2634 (1997).
Gerber, H. P., Dixit, V. & Ferrara, N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 273, 13313–13316 (1998).
Förstermann, U. et al. Isoforms of nitric oxide synthase. Characterization and purification from different cell types. Biochem. Pharmacol. 42, 1849–1857 (1991).
Yano, S., Tokumitsu, H. & Soderling, T. R. Calcium promotes cell survival through CaM-K kinase activation of the protein kinase-B pathway. Nature 396, 584–587 (1998).
Zeiher, A. M., Fisslthaler, B., Schray-Utz, B. & Busse, R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ. Res. 76, 980–986 (1995).
Khwaja, A., Rodriquez-Viciana, P., Wennström, S., Warne, P. H. & Downward, J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 16, 2783–2793 (1997).
Dimmeler, S., Haendeler, J., Nehls, M. & Zeiher, A. M. Suppression of apoptosis by nitric oxide via inhibition of ICE-like and CPP32-like proteases. J. Exp. Med. 185, 601–608 (1997).
Kauffmann-Zeh, A. et al. Suppression of c-Myc-induced apoptosis by Ras signalling through PI(3)K and PKB. Nature 385, 544–548 (1997).
Copper, J. A., Sefton, B. M. & Hunter, T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 99, 387–402 (1983).
Acknowledgements
We thank C. Goebel, I. Winter and S. Ficus for expert technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dimmeler, S., Fleming, I., Fisslthaler, B. et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399, 601–605 (1999). https://doi.org/10.1038/21224
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/21224
This article is cited by
-
Apelin-VEGF-C mRNA delivery as therapeutic for the treatment of secondary lymphedema
EMBO Molecular Medicine (2024)
-
Optimizing irradiation conditions for low-intensity pulsed ultrasound to upregulate endothelial nitric oxide synthase
Journal of Medical Ultrasonics (2024)
-
Estrogen-receptor status determines differential regulation of α1- and α2-adrenoceptor-mediated cell survival, angiogenesis, and intracellular signaling responses in breast cancer cell lines
Medical Oncology (2024)
-
Effects and Optimal Dose of Exercise on Endothelial Function in Patients with Heart Failure: A Systematic Review and Meta-Analysis
Sports Medicine - Open (2023)
-
Cold-induced vasodilation response in a Japanese cohort: insights from cold-water immersion and genome-wide association studies
Journal of Physiological Anthropology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.