Abstract
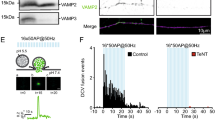
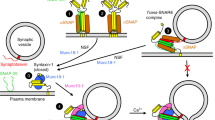
The temporal sequence of SNARE protein interactions that cause exocytosis is unknown. Blockade of synaptic neurotransmitter release through cleavage of VAMP/synaptobrevin by tetanus toxin light chain (TeNT-LC) was accelerated by nerve stimulation. Botulinum/B neurotoxin light chain (BoNT/B-LC), which cleaves VAMP at the same site as TeNT-LC, did not require stimulation. Because TeNT-LC requires the N-terminal coil domain of VAMP for binding but BoNT/B-LC requires the C-terminal coil domain, it seems that, before nerve activity, the N-terminal domain is shielded in a protein complex, but the C-terminal domain is exposed. This N-terminal complex lasts until nerve activity occurrs and may serve to cock synaptic vesicles for immediate exocytosis upon Ca2+ entry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Söllner, T., Bennett, M. K., Whiteheart, S. W., Scheller, R. H. & Rothman, J. E. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75, 409–418 (1993).
Weis, W. I. & Scheller, R. H. SNARE the rod, coil the complex. Nature 395, 328–329 (1998).
Rizo, J. & Südhof, T. C. Mechanics of membrane fusion. Nat. Struct. Biol. 5, 839–842 (1998).
Ryan, T. A. Probing a complex question: when are SNARE proteins ensnared? Nat. Neurosci. 1, 175–177 (1998).
Hayashi, T. et al. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 13, 5051–5061 (1994).
Hua, S.-Y., Raciborska, D. A., Trimble, W. S. & Charlton, M. P. Different VAMP/synaptobrevin complexes for spontaneous and evoked transmitter release at the crayfish neuromuscular junction. J. Neurophysiol. 80, 3233–3247 (1998).
Ferrer-Montiel, A. V., Canaves, J. M., DasGupta, B. R., Wilson, M. C. & Montal, M. Tyrosine phosphorylation modulates the activity of clostridial neurotoxins. J. Biol. Chem. 271, 18322–18325 (1996).
Schiavo, G. et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359, 832–835 (1992).
Cornille, F. et al. Cooperative exosite-dependent cleavage of synaptobrevin by tetanus toxin light chain. J. Biol. Chem. 272, 3459–3464 (1997).
Pellizzari, R. et al. Structural determinants of the specificity for synaptic vesicle-associated membrane protein/synaptobrevin of tetanus and botulinum type B and G neurotoxins. J. Biol. Chem. 271, 20353–20358 (1996).
Rossetto, O. et al. SNARE motif and neurotoxins. Nature 372, 415–416 (1994).
Pellizzari, R., Mason, S., Shone, C. C. & Montecucco, C. The interaction of synaptic vesicle-associated membrane protein/synaptobrevin with botulinum neurotoxins D and F. FEBS Lett. 409, 339–342 (1997).
Sheng, Z. H., Rettig, J., Cook, T. & Catterall, W. A. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature 379, 451–454 (1996).
Llinas, R., Steinberg, I. Z. & Walton, K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys. J. 33, 323–351 (1981).
Poirier, M. A. et al. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat. Struct. Biol. 5, 765–769 (1998).
Sutton, R. B., Fasshauer, D., Jahn, R. & Brunger, A. T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395, 347–353 (1998).
Chen, Y. A., Scales, S. J., Patel, S. M., Doung, Y.-C. & Scheller, R. H. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell 97, 165–174 (1999).
Hayashi, T., Yamasaki, S., Nauenburg, S., Binz, T. & Niemann, H. Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J. 14, 2317–2325 (1995).
Regazzi, R. et al. Mutational analysis of VAMP domains implicated in Ca2+-induced insulin exocytosis. EMBO J. 15, 6951–6959 (1996).
Fiebig, K. M., Rice, L. M., Pollock, E. & Brunger, A. T. Folding intermediates of SNARE complex assembly. Nat. Struct. Biol. 6, 117–123 (1999).
Calakos, N. & Scheller, R. H. Vesicle-associated membrane protein and synaptophysin are associated on the synaptic vesicle. J. Biol. Chem. 269, 24534–24537 (1994).
Washbourne, P., Schiavo, G. & Montecucco, C. Vesicle-associated membrane protein-2 (synaptobrevin-2) forms a complex with synaptophysin. Biochem. J. 305, 721–724 (1995).
McMahon, H. T. et al. Synaptophysin, a major synaptic vesicle protein, is not essential for neurotransmitter release. Proc. Natl. Acad. Sci. USA 93, 4760–4764 (1996).
Otto, H., Hanson, P. I. & Jahn, R. Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin, and SNAP-25 in the membrane of synaptic vesicles. Proc. Natl. Acad. Sci. USA 94, 6197–6201 (1997).
Xu, T., Binz, T., Niemann, H. & Neher, E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat. Neurosci. 1, 192–200 (1998).
Edwards, R. H. Neurotransmitter release: variations on a theme. Curr. Biol. 8, R883–885 (1998).
Morgan, A. & Burgoyne, R. D. Common mechanisms for regulated exocytosis in the chromaffin cell and the synapse. Semin. Cell Dev. Biol. 8, 141–149 (1997).
Burgoyne, R. D., Geisow, M. J. & Barron, J. Dissection of stages in exocytosis in the adrenal chromaffin cell with use of trifluoperazine. Proc. R. Soc. Lond. B Biol. Sci. 216, 111–115 (1982).
Kasai, H. Comparative biology of Ca2+-dependent exocytosis: implications of kinetic diversity for secretory function. Trends Neurosci. 22, 88–93 (1999).
Schweizer, F. E. et al. Regulation of neurotransmitter release kinetics by NSF. Science 279, 1203–1206 (1998).
Burke, R. E. & Rudomin, P. in Handbook of Physiology section 1 (eds. Brookhart, J. M., Mountcastle, V. M., Kandel, E. R. & Geiger, S. R.) 877–944 (Williams and Wilkins, Baltimore, 1977).
Matteoli, M. et al. Synaptic vesicle endocytosis mediates the entry of tetanus neurotoxin into hippocampal neurons. Proc. Natl. Acad. Sci. USA 93, 13310–13315 (1996).
Acknowledgements
We thank W. Trimble for TeNT-LC and BoNT/D-LC, A. Zdanovsky for BoNT/B-LC, C. C. Shone for anti-VAMP and H. Bellen for anti-synaptotagmin. We thank M. Salter for assistance with the phosphotyrosine assay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hua, SY., Charlton, M. Activity-dependent changes in partial VAMP complexes during neurotransmitter release. Nat Neurosci 2, 1078–1083 (1999). https://doi.org/10.1038/16005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/16005
This article is cited by
-
Unzipping of neuronal snare protein with steered molecular dynamics occurs in three steps
Journal of Molecular Modeling (2014)
-
Complexin arrests a pool of docked vesicles for fast Ca2+-dependent release
The EMBO Journal (2012)
-
SNARE Requirements En Route to Exocytosis: from Many to Few
Journal of Molecular Neuroscience (2012)
-
Complexin cross-links prefusion SNAREs into a zigzag array
Nature Structural & Molecular Biology (2011)
-
Complexin activates and clamps SNAREpins by a common mechanism involving an intermediate energetic state
Nature Structural & Molecular Biology (2011)