Abstract
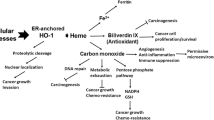
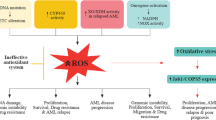
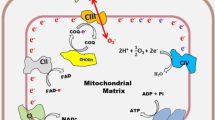
Heme oxygenase-1 (HO-1) is an inducible enzyme that catalyzes oxidative degradation of heme to form biliverdin, carbon monoxide (CO), and free iron. Biliverdin is subsequently reduced to bilirubin by the enzyme biliverdin reductase. Increasing evidence has indicated the critical role of HO-1 in cytoprotection and more diverse biological functions. Induction of HO-1 by various chemical inducers that are primarily cell stress inducers or by HO-1 gene transfection confers a protective capacity to cultured cells as well as to cells in several in vivo animal models. In addition, HO-1-deficient mice exhibit a significant increase in susceptibility to tissue injury. The cytoprotective action of HO-1 seems to be mainly a function of the antiapoptotic effects of the enzyme. HO-1 is believed to exert this antiapoptotic action by multiple mechanisms: (a) decreased intracellular pro-oxidant levels, (b) increased bilirubin levels, and (c) elevated CO production. CO may produce an antiapoptotic effect by inhibiting both expression of p53 and release of mitochondrial cytochrome c. HO-1 may also be a target in antitumor therapy because the growth of most tumors depends on HO-1. Our preliminary studies with an HO inhibitor showed a promising antitumor effect. This preliminary work warrants continued investigation for possible novel anticancer chemotherapy.
Similar content being viewed by others
References
Maines MD. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 1988; 2: 2557–2568.
Shibahara S. Regulation of heme oxygenase gene expression. Semin Hematol 1988; 25: 370–376.
Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: A major physiologic cytoprotectant. Proc Natl Acad Sci USA 2002; 99: 16093–16098.
McCoubrey WK Jr, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem 1997; 247: 725–732.
Motterlini R, Foresti R, Bassi R, Calabrese V, Clark JE, Green CJ. Endothelial heme oxygenase-1 induction by hypoxia. Modulation by inducible nitric oxide synthase and S-nitrosothiols. J Biol Chem 2000; 275: 13613–13620.
Mitani K, Fujita H, Fukuda Y, Kappas A, Sassa S. The role of inorganic metals and metalloporphrins in the induction of heme oxygenase and heat-shock protein 70 in human hepatoma cells. Biochem J 1993; 290: 819–825.
Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Aci USA 1989; 86: 99–103.
Hara E, Takahashi K, Takeda K, et al. Induction of heme oxygenase-1 as a response in sensing the signals evoked by distinct nitric oxide donors. Biochem Pharmacol 1999; 58: 227–236.
Hartsfield SL, Alam J, Cook JL, Choi AMK. Regulation of heme oxygenase-1 gene expression in vascular smooth muscle cells by nitric oxide. Am J Physiol 1997; 273: L980–L988.
Doi K, Akaike T, Fujii S, et al. Induction of haem oxygenase-1 by nitric oxide and ischaemia in experimental solid tumours and implications for tumour growth. Br J Cancer 1999; 80: 1945–1954.
Tanaka S, Akaike T, Fang J, et al. Antiapoptotic effect of heme oxygenase-1 induced by nitric oxide in experimental solid tumor. Br J Cancer 2003; 88: 902–909.
Zamora R, Vodovotz Y, Aulak KS, et al. A DNA microarray study of nitric oxide-induced genes in mouse hepatocytes: Implications for hepatic hemeoxygenase-1 expression in ischemia/reperfusion. Nitric Oxide 2002; 7: 165–186.
Stocker R. Induction of heme oxygenase as a defence against oxidative stress. Free Radic Res Commun 1990; 9: 101–112.
Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: A novel target for the modulation of the inflammatory response. Nat Med 1996; 2: 87–90.
Siow RC, Sato H, Mann GE. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: Anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc Res 1999; 41: 385–394.
Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol 2000; 60: 1121–1128.
Durante W. Carbon monoxide and bile pigments: Surprising mediators of vascular function. Vasc Med 2002; 7: 195–202.
Hill-Kapturczak N, Chang SH, Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol 2002; 21: 307–321.
Sato K, Balla J, Otterbein L, et al. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol 2001; 166: 4185–4196.
Wagner M, Cadetg P, Ruf R, Mazzucchelli L, Ferrari P, Redaelli CA. Heme oxygenase-1 attenuates ischemia/reperfusion-induced apoptosis and improves survival in rat renal allografts. Kidney Int 2003; 63: 1564–1573.
Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: A protective role in cisplatin nephrotoxicity in the rat. Kidney Int 1995; 48: 1298–1307.
Lee TS, Chau LY. Heme oxygenase-1 mediates the antiinflammatory effect of interleukin-10 in mice. Nat Med 2002; 8: 240–246.
Tullius SG, Nieminen-Kelha M, Buelow R, et al. Inhibition of ischemia/reperfusion injury and chronic graft deterioration by a single-donor treatment with cobalt-protoporphyrin for the induction of heme oxygenase-1. Transplantation 2002; 74: 591–598.
Abraham NG, Lavrovsky Y, Schwartzman ML, et al. Transfection of the human heme oxygenase gene into rabbit coronary microvessel endothelial cells: Protective effect against heme and hemoglobin toxicity. Proc Natl Acad Sci USA 1995; 92: 6798–6802.
Amersi F, Buelow R, Kato H, et al. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest 1999; 104: 1631–1639.
Otterbein LE, Kolls JK, Mantell LL, Cook JL, Alam J, Choi AM. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J Clin Invest 1999; 103: 1047–1054.
Panahian N, Yoshiura M, Maines MD. Over expression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem 1999; 72: 1187–1203.
Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol 2000; 278: H643–H651.
Hangaishi M, Ishizaka N, Aizawa T, et al. Induction of heme oxygenase-1 can act protectively against cardiac ischemia/reperfusion in vivo. Biochem Biophys Res Commun 2000; 279: 582–588.
Yet SF, Tian R, Layne MD, et al. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 2001; 89: 168–173.
Coito AJ, Buelow R, Shen XD, et al. Heme oxygenase-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from ischemia-reperfusion injury. Transplantation 2002; 74: 96–102.
Hirai H, Kubo H, Yamaya M, et al. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood 2003; 102: 1619–1621.
Choi BM, Pae HO, Chung HT. Nitric oxide priming protects nitric oxide-mediated apoptosis via heme oxygenase-1 induction. Free Radic Biol Med 2003; 34: 1136–1145.
Amon M, MengerMD, Vollmar B. Heme oxygenase and nitric oxide synthase mediate cooling-associated protection against TNF-?-induced microcirculatory dysfunction and apoptotic cell death. FASEB J 2003; 17: 175–185.
Ke B, Shen XD, Zhai Y, et al. Heme oxygenase 1 mediates the immunomodulatory and antiapoptotic effects of interleukin 13 gene therapy in vivo and in vitro. Hum Gene Ther 2002; 13: 1845–1857.
Vulapalli SR, Chen Z, Chua BH, Wang T, Liang CS. Cardioselective overexpression of HO-1 prevents I/R-induced cardiac dysfunction and apoptosis. Am J Physiol 2002; 283: H688–H694.
Clark JE, Green CJ, Motterlini R. Involvement of the heme oxygenase-carbon monoxide pathway in keratinocyte proliferation. Biochem Biophys Res Commun 1997; 241: 215–220.
Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem 1998; 68: 121–127.
Li Volti G, Wang J, Traganos F, Kappas A, Abraham NG. Differential effect of heme oxygenase-1 in endothelial and smooth muscle cell cycle progression. Biochem Biophys Res Commun 2002; 296: 1077–1082.
Hanselmann C, Mauch C, Werner S. Heme oxygenase-1: A novel player in cutaneous wound repair and psoriasis? Biochem J 2001; 353: 459–466.
Goodman AI, Choudhury M, da Silva JL, Schwartzman ML, Abraham NG. Overexpression of the heme oxygenase gene in renal cell carcinoma. Proc Soc Exp Biol Med 1997; 214: 54–61.
Tsuji MH, Yanagawa T, Iwasa S, et al. Heme oxygenase-1 expression in oral squamous cell carcinoma as involved in lymph node metastasis. Cancer Lett 1999; 138: 53–59.
Deininger MH, Meyermann R, Trautmann K, et al. Heme oxygenase (HO)-1 expressing macrophages/microglial cells accumulate during oligodendroglioma progression. Brain Res 2000; 882: 1–8.
Torisu-Itakura H, Furue M, Kuwano M, Ono M. Coexpression of thymidine phosphorylase and heme oxygenase-1 in macrophages in human malignant vertical growth melanomas. Jpn J Cancer Res 2000; 91: 906–910.
Sahoo SK, Sawa T, Fang J, et al. Pegylated zinc protoporphyrin: A water-soluble heme oxygenase inhibitor with tumor-targeting capacity. Bioconjugate Chem 2002; 13: 1031–1038.
Fang J, Sawa T, Akaike T, et al. In vivo antitumor activity of pegylated zinc protoporphyrin: Targeted inhibition of heme oxygenase in solid tumor. Cancer Res 2003; 63: 3567–3574.
Kato H, Amersi F, Buelow R, et al. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant 2001; 1: 121–128.
Salahudeen AA, Jenkins JK, Huang H, Ndebele K, Salahudeen AK. Overexpression of heme oxygenase protects renal tubular cells against cold storage injury: Studies using hemin induction and HO-1 gene transfer. Transplantation 2001; 72: 1498–1504.
Pileggi A, Molano RD, Berney T, et al. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes 2001; 50: 1983–1991.
Takeda A, Perry G, Abraham NG, et al. Overexpression of heme oxygenase in neuronal cells, the possible interaction with Tau. J Biol Chem 2000; 275: 5395–5399.
Dennery PA, Sridhar KJ, Lee CS, et al. Heme oxygenasemediated resistance to oxygen toxicity in hamster fibroblasts. J Biol Chem 1997; 272: 14937–14942.
Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA 1997; 94: 10919–10924.
Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA 1997; 94: 10925–10930.
Yachie A, Niida Y, Wada T, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 1999; 103: 129–135.
Tulis DA, Durante W, Liu X, Evans AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation 2001; 104: 2710–2715.
Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation 2002; 105: 79–84.
Akaike T, Sato K, Ijiri S, Miyamoto Y, et al. Bactericidal activity of alkyl peroxyl radicals generated by heme-iron-catalyzed decomposition of organic peroxides. Arch Biochem Biophys 1992; 294: 55–63.
Sawa T, Akaike T, Kida K, Fukushima Y, Takagi K, Maeda H. Lipid peroxyl radicals from oxidized oils and heme-iron: Implication of a high-fat diet in colon carcinogenesis. Cancer Epidemiol Biomarkers Prev 1998; 7: 1007–1012.
Kanazawa A, Sawa T, Akaike T, Morimur S, Kida K, Maeda H. Generation of lipid peroxyl radicals from edible oils and their biological activities: A need for consideration for anti-radical components and purification processing. Biofactors 2000; 13: 187–193.
Jeney V, Balla J, Yachie A, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood 2002; 100: 879–887.
Vile GF, Tyrrell RM. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem 1993; 268: 14678–14681.
Ferris CD, Jaffrey SR, Sawa A, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol 1999; 1: 152–157.
Dumont A, Hehner SP, Hofmann TG, Ueffing M, DrogeW, Schmitz ML. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-?B. Oncogene 1999; 18: 747–757.
Slater AF, Stefan C, Nobel I, van den Dobbelsteen DJ, Orrenius S. Signalling mechanisms and oxidative stress in apoptosis. Toxicol Lett 1995; 82/83: 149–153.
Esteve JM, Mompo J, Garcia de la Asuncion J, et al. Oxidative damage to mitochondrial DNA and glutathione oxidation in apoptosis: Studies in vivo and in vitro. FASEB J 1999; 13: 1055–1064.
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993; 75: 241–251.
Johnson TM, Yu ZX, Ferrans VJ, Lowenstein RA, Finkel T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci USA 1996; 93: 11848–11852.
Korsmeyer SJ. Regulators of cell death. Trends Genet 1995; 11: 101–105.
Um HD, Orenstein JM, Wahl SM. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol 1996; 156: 3469–3477.
Zamzami N, Marchetti P, Castedo M, et al. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 1995; 182: 367–377.
Gourley GR. Bilirubin metabolism and kernicterus. Adv Pediatr 1997; 44: 173–229.
Kimura M, Matsumura Y, Konno T, Miyauchi Y, Maeda H. Enzymatic removal of bilirubin toxicity by bilirubin oxidase in vitro and excretion of degradation products in vivo. Proc Soc Exp Biol Med 1990; 195: 64–69.
Minetti M, Mallozzi C, Di Stasi AM, Pietraforte D. Bilirubin is an effective antioxidant of peroxynitrite-mediated protein oxidation in human blood plasma. Arch Biochem Biophys 1998; 352: 165–174.
Dore S, Takahashi M, Ferris CD, et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci USA 1999; 96: 2445–2450.
Dore S, Sampei K, Goto S, et al. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol Med 1999; 5: 656–663.
Dore S, Goto S, Sampei K, et al. Heme oxygenase-2 acts to prevent neuronal death in brain cultures and following transient cerebral ischemia. Neuroscience 2000; 99: 587–592.
Suematsu M, Kashiwagi S, Sano T, Goda N, Shinoda Y, Ishimura Y. Carbon monoxide as an endogenous modulator of hepatic vascular perfusion. Biochem Biophys Res Commun 1994; 205: 1333–1337.
Petrache I, Otterbein LE, Alam J, Wiegand GW, Choi AM. Heme oxygenase-1 inhibits TNF-?-induced apoptosis in cultured fibroblasts. Am J Physiol 2000; 278: L312–L319.
Brouard S, Otterbein LE, Anrather J, et al. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 2000; 192: 1015–1026.
Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc Res 2002; 55: 396–405.
Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol 1999; 276: L688–L694.
Inguaggiato P, Gonzalez-Michaca L, Croatt AJ, Haggard JJ, Alam J, Nath KA. Cellular overexpression of heme oxygenase-1 up-regulates p21 and confers resistance to apoptosis. Kidney Int 2001; 60: 2181–2191.
Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol 1998; 152: 711–720.
Ishikawa K, Navab M, Leitinger N, Fogelman AM, Lusis AJ. Induction of heme oxygenase-1 inhibits monocyte transmigration induced by mildly oxidized LDL. J Clin Invest 1997; 100: 1209–1216.
Ishizaka N, Leon HD, Laursen JB, et al. Angiotensin II-induced hypertension increases heme oxygenase-1 expression in rat aorta. Circulation 1997; 96: 1923–1929.
Nath KA, Balla G, Vercellotti GM, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest 1992; 90: 267–270.
Yamada N, Yamaya M, Okinaga S, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet 2000; 66: 187–195.
Guo X, Shin VY, Cho CH. Modulation of heme oxygenase in tissue injury and its implication in protection against gastrointestinal diseases. Life Sci 2001; 69: 3113–3119.
Smith MA, Kutty RK, Richey PL, et al. Heme oxygenase-1 is associated with the neurofibrillary pathology of Alzheimer's disease. Am J Pathol 1994; 145: 42–47.
Ishizuka K, Kimura T, Yoshitake J, et al. Possible assessment for antioxidant capacity in Alzheimer's disease by measuring lymphocyte heme oxygenase-1 expression with real-time RT-PCR. Ann N Y Acad Sci 2002; 977: 173–178.
Levere RD, Staudinger R, Loewy G, Kappas A, Shibahara S, Abraham NG. Elevated levels of heme oxygenase-1 activity and mRNA in peripheral blood adherent cells of acquired immunodeficiency syndrome patients. Am J Hematol 1993; 43: 19–23.
Sato H, Siow RC, Bartlett S, et al. Expression of stress proteins heme oxygenase-1 and-2 in acute pancreatitis and pancreatic islet ?TC3 and acinar AR42J cells. FEBS Lett 1997; 405: 219–223.
Chen W, Hunt DM, Lu H, Hunt RC. Expression of antioxidant protective proteins in the rat retina during prenatal and postnatal development. Invest Ophthalmol Vis Sci 1999; 40: 744–751.
Laniado-Schwartzman M, Abraham NG, Conners M, Dunn MW, Levere RD, Kappas A. Heme oxygenase induction with attenuation of experimentally induced corneal inflammation. Biochem Pharmacol 1997; 53: 1069–1075.
Horvath I, Donnelly LE, Kiss A, Paredi P, Kharitonov SA, Barnes PJ. Raised levels of exhaled carbon monoxide are associated with an increased expression of heme oxygenase-1 in airway macrophages in asthma: A new marker of oxidative stress. Thorax 1998; 53: 668–672.
Mautes AE, Kim DH, Sharp FR, et al. Induction of heme oxygenase-1 (HO-1) in the contused spinal cord of the rat. Brain Res 1998; 795: 17–24.
Matz PG, Weinstein PR, Sharp FR. Heme oxygenase-1 and heat shock protein 70 induction in glia and neurons throughout rat brain after experimental intracerebral hemorrhage. Neurosurgery 1997; 40: 152–160.
Greenstein JP. Biochemistry of Cancer, 2nd edn. New York: Academic Press, 1954: 518–541.
Sato K, Ito K, Kohara H, Yamaguchi Y, Adachi K, Endo H. Negative regulation of catalase gene expression in hepatoma cells. Mol Cell Biol 1992; 12: 2525–2533.
Hasegawa Y, Takano T, Miyauchi A, et al. Decreased expression of glutathione peroxidase mRNA in thyroid anaplastic carcinoma. Cancer Lett 2002; 182: 69–74.
Yamanaka N, Deamer D. Superoxide dismutase activity in WI-38 cell cultures: Effects of age, trypsinization and SV-40 transformation. Physiol Chem Phys 1974; 6: 95–106.
Matsushita Y, Kumagai H, Yoshimoto A, et al. Antitumor activities of (2”R)-4'-O-tetrahydropyranyl-adriamycin (THP) and its combination with other antitumor agents on murine tumors. J Antibiot (Tokyo) 1985; 38: 1408–1419.
Fang J, Sawa T, Akaike T, Greish K, Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer 2003 (in press).
Simizu S, Takada M, Umezawa K, Imoto M. Requirement of caspase-3(-like) protease-mediated hydrogen peroxide production for apoptosis induced by various anticancer drugs. J Biol Chem 1998; 273: 26900–26907.
Fang J, Sawa T, Akaike T, Maeda H. Tumor-targeted delivery of polyethylene glycol-conjugated D-amino acid oxidase for antitumor therapy via enzymatic generation of hydrogen peroxide. Cancer Res 2002; 62: 3138–3143.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986; 46: 6387–6392.
Maeda H. SMANCS and polymer-conjugated macromolecular drugs: Advantages in cancer chemotherapy. Adv Drug Deliv Rev 2001; 46: 169–185.
Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul 2001; 41: 189–207.
Maeda H, Sawa T, Konno T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release 2001; 74: 47–61.
Fang J, Sawa T, Maeda H. Factors and mechanism of "EPR" effect and the enhanced antitumor effects of macromolecular drugs including SMANCS. Adv Exp Med Biol 2003; 519: 29–49.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, J., Akaike, T. & Maeda, H. Antiapoptotic role of heme oxygenase (HO) and the potential of HO as a target in anticancer treatment. Apoptosis 9, 27–35 (2004). https://doi.org/10.1023/B:APPT.0000012119.83734.4e
Issue Date:
DOI: https://doi.org/10.1023/B:APPT.0000012119.83734.4e