Abstract
Purpose. To explore the use of cyclodextrins (CD) to form inclusion complexes with β-lapachone (β-lap) to overcome solubility and bioavailability problems previously noted with this drug.
Methods. Inclusion complexes between β-lap and four cyclodextrins (α-, β-, γ-, and HPβ-CD) in aqueous solution were investigated by phase solubility studies, fluorescence, and 1H-NMR spectroscopy. Biologic activity and bioavailability of β-lap inclusion complexes were investigated by in vitro cytotoxicity studies with MCF-7 cells and by in vivo lethality studies with C57Blk/6 mice (18-20 g).
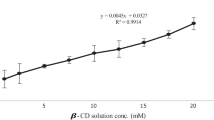
Results. Phase solubility studies showed that β-lap solubility increased in a linear fashion as a function of α-, β-, or HPβ-CD concentrations but not γ-CD. Maximum solubility of β-lap was achieved at 16.0 mg/ml or 66.0 mM with HPβ-CD. Fluorescence and 1H-NMR spectroscopy proved the formation of 1:1 inclusion complexes between β-CD and HPβ-CD with β-lap. Cytotoxicity assays with MCF-7 cells showed similar biologic activities of β-lap in β-CD or HPβ-CD inclusion complexes (TD50 = 2.1 μM). Animal studies in mice showed that the LD50 value of β-lap in an HPβ-CD inclusion complex is between 50 and 60 mg/kg.
Conclusions. Complexation of β-lap with HPβ-CD offers a major improvement in drug solubility and bioavailability.
Similar content being viewed by others
References
A. Marin, A. Lopex de Cerain, E. Hamilton, A. D. Lewis, J. M. Martinez-Penuela, M. A. Idoate, and J. Bello. DT-diaphorase and cytochrome B5 reductase in human lung and breast tumors. Br. J. Cancer 76:923-929 (1997).
A. M. Malkinson, D. Siegel, G. L. Forrest, A. F. Gazdar, H. K. Oie, D. C. Chan, P. A. Bunn, M. Mabry, D. J. Dykes, S. D. Harrison, and D. Ross. Elevated DT-diaphorase activity and messenger RNA content in human non-small cell lung carcinoma: relationship to the response of lung tumor xenografts to mitomycin Cl. Cancer Res. 52:4752-4757 (1992).
A. M. Malkinson. Molecular comparison of human and mouse pulmonary adenocarcinomas. Exp. Lung Res. 24:541-555 (1998).
M. Belinsky and A. K. Jaiswal. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 12(2):103-117 (1993).
V. J. Stella and R. A. Rajewski. Cyclodextrins: Their future drug formulation and delivery. Pharm. Res. 14:556-567 (1997).
J. Szejtli. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98:1743-1753 (1998).
T. Loftsson and M. E. Brewster. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization, J Pharm Sci. 85:1017-1025 (1996).
R. A. Rajewski and V. J. Stella. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J. Pharm. Sci. 85:1142-1169 (1996).
T. Irie and K. Uekama. Pharmaceutical applications of cyclodextrins. 3. Toxicological issues and safety evaluation. J. Pharm. Sci. 86:147-162 (1997).
M. V. Rekharsky and Y. Inoue. Complexation thermodynamics of cyclodextrin. Chem. Rev. 98:1875-1917 (1998).
J. L. Lach and T. F. Chin. Interaction of pharmaceuticals with Schardinger dextrins III. J. Pharm. Sci. 53:69-73 (1964).
S. M. Planchon, S. Wuerzberger, B. Frydman, D. T. Witiak, P. Hutson, D. R. Church, G. Wilding, and D. A. Boothman. Beta-lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: a p53-independent response. Cancer Res. 55:3706-3711 (1995).
J. J. Pink, S. M. Planshon, C. Tagliarino, S. M. Wuerzberger-Davis, M. E. Varnes, D. Siegel, and D. A. Boothman. NAD(P)H:quinone oxidoreductase (NQO1) activity is the principal determinant of beta-lapachone cytoxicity. J. Biol. Chem. 275:5416-5424 (2000).
T. Higuchi and K. A. Connors. Phase solubility techniques. Adv. Anal. Chem. Instrum. 4:117-212 (1965).
A. Botsi, K. Yannakopoulou, B. Perly, and E. Hadjoudis. Positives or adverse effects of methylation on the inclusion behavior of cyclodextrins. A comparative NMR study using pheromone constituents of the olive fruit fly. J. Org. Chem. 60:4017-4023 (1995).
S. M. Wuerzberger, J. J. Pink, S. M. Planchon, K. L. Byers, W. G. Bornmann, and D. A. Boothman. Induction of apoptosis in MCF-7:WS8 breast cancer cells by beta-lapachone. Cancer Res. 58:1876-1885 (1998).
C. Labarca and K. Paigen. A simple, rapid, and sensitive DNA assay procedure. Anal. Biochem. 102:344-352 (1980).
K. A. Connors. The stability of cyclodextrin complexes in solution. Chem. Rev. 97:1325-1357 (1997).
P. Adell, T. Parella, F. Sanchez-Ferrando, and A. Virgili. Clean selective spin-locking spectra using pulsed field gradients. J. Magn. Reson. 108:77-80 (1995).
Y. Ikeda, S. Motoune, T. Matsuoka, H. Arima, F. Hirayama, and K. Uekama. Inclusion complex formation of captopril with α-and β-cyclodextrins in aqueous solution: NMR spectroscopic and molecular dynamic studies. J. Pharm. Sci. 91:2390-2398 (2002).
V. Ramamurthy and D. F. Eaton. Photochemistry and photophysics within cyclodextrin cavities. Acc. Chem. Res. 21:300-306 (1988).
E. E. Sideris, G. N. Valsami, M. A. Koupparis, and P. E. Macheras. Determination of association constants in cyclodextrin/drug complexation using the Scatchard plot: application to β-cyclodextrin anilinonaphthalenesulfonates. Pharm. Res. 9:1568-1574 (1992).
C. Tagliarino, J. J. Pink, G. R. Dubyak, A. L. Nieminen, and D. A. Boothman. Calcium is a key signaling molecule in beta-lapachone-mediated cell death. J. Biol. Chem. 276:19150-19159 (2001).
C. J. Li, Y. Z. Li, A. V. Pinto, and A. B. Pardee. Potent inhibition of tumor survival in vivo by beta-lapachone plus taxol: combining drugs imposes different artificial checkpoints. Proc. Natl. Acad. Sci. USA 96:13369-13374 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nasongkla, N., Wiedmann, A.F., Bruening, A. et al. Enhancement of Solubility and Bioavailability of β-Lapachone Using Cyclodextrin Inclusion Complexes. Pharm Res 20, 1626–1633 (2003). https://doi.org/10.1023/A:1026143519395
Issue Date:
DOI: https://doi.org/10.1023/A:1026143519395