Abstract
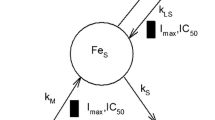
Basic physiologic indirect response models have been proposed to account for the pharmacodynamics of drugs that act by way of inhibition or stimulation of the production or loss of endogenous substances or mediators. In this work, these models were applied to account for the effects of recombinant human erythropoietin (rHuEpo) in man. Indeed, rHuEpo induces a delayed increase of serum soluble transferrin receptors (sTfr) and a delayed decrease in ferritin (fr) concentrations. The purpose of the present study was to compare two pharmacodynamic approaches to relate serum erythropoietin (Epo) concentrations to the effect of rHuEpo on sTfr, and fr, the “indirect effect” and the “effect compartment” models. However, due to the average lag lime of about 50 hr between the first intake of rHuEpo and the onset of the measurable effects, a delay function was incorporated into the “indirect response models” to describe the relationship between the Epo plasma concentrations and the endogenous receptors or mediators affected by the drug and responsible for the effects on sTfr and fr. There are no real differences in the descriptive features of the two models used. For these reasons, the indirect model seems more appropriate because it supplies a possible mechanistic interpretation of the physiological process.
Similar content being viewed by others
REFERENCES
B. Berglund and B. Ekblom. Effect of recombinant human erythropoietin treatment on blood pressure and some haematological parameters in healthy males. J. Int. Med. 229:125–130 (1991).
B. Ekblom and B. Berglund. Effect of erythropoietin administration on maximal aerobic power in man. Scand. J. Med. Sci. Sports 1:88–93 (1991).
A. T. Kicman and D. A. Cowan. Peptides hormones and sport: Misuse and detection. Br. Med. Bull. 48:496–517 (1992).
M. J. Koury and M. C. Bondurant. The molecular mechanism of erythropoietin action. Eur. J. Biochem. 210:649–663 (1992).
W. Jelkman. Biology of erythropoietin. Clin. Invest. 72:S3–S10 (1994).
R. Gareau, M. Audran, R. D. Baynes, C. H. Flowers, A. Duvallet, L. Senécal, and G. R. Brisson. Erythropoietin abuse in athletes. Nature 380:113 (1996).
F. Bressolle, M. Audran, R. Gareau, R. D. Baynes, C. Guiolicelli, and R. Gomeni. Population pharmacodynamics for monitoring epoetin in athletes Clin. Drug. Invest. 14:233–242.
A. Souillard, M. Audran, F. Bressolle, R. Gareau, A. Duvallet, and J. L. Chanal. Recombinant human erythropoietin and pharmacodynamics parameters in athletes. Interest of blood sampling for doping control. Br. J. Clin. Pharmacol. 42:355–360 (1996).
L. B. Sheiner, D. R. Stanski, S. Vozeh, R. D. Miller, and J. Ham. Simultaneous modelling of pharmacokinetics and pharmacodynamics: Application to d-tubocurarine. Clin. Pharmacol. Ther. 25:358–371 (1979).
W. J. Jusko and H. C. Ko. Physiologic indirect response models characterize diverse types of pharmacodynamic effects. Clin. Pharmacol. Ther. 56:406–419 (1994).
N. L. Dayneka, V. Garg, and W. J. Jusko. Comparison of four basic models of indirect pharmacodynamic responses. J. Pharmacokin. Biopharm. 21:457–478 (1993).
D. V. Verotta and L. B. Sheiner. A general conceptual model for non-steady state pharmacokinetic/pharmacodynamic data. J. Pharmacokin Biopharm. 23:1–4 (1995).
W. J. Jusko, H. C. Ko, and W. F. Ebling. Convergence of direct and indirect pharmacodynamic response models. J. Pharmacokin. Biopharm. 23:5–10 (1995).
L. Aarons. The estimation of population pharmacokinetic parameters using an EM algorithm. Comput. Meth. Prog. Biomed. 41:9–16 (1993).
EM-fit computer program, version 1.0, Bestfit, Luxembourg, 1996.
R. R. Sokal and J. R. N. Rohlf. Biometry, W. H. Freeman, San Francisco, 1969.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bressolle, F., Audran, M., Gareau, R. et al. Comparison of a Direct and Indirect Population Pharmacodynamic Model: Application to Recombinant Human Erythropoietin in Athletes. J Pharmacokinet Pharmacodyn 25, 263–275 (1997). https://doi.org/10.1023/A:1025737024403
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1025737024403