Abstract
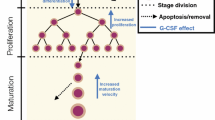
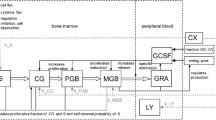
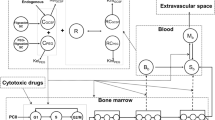
The leukopenic effects of anticancer agents are described using a semi-physiologic multiple-pool cell lifespan model. The time course of myelosuppression in relation to the drug concentration vs. time profile was characterized using a three pool indirect model. The proliferation and maturation stages of myeloid cells in the bone marrow and cell removal from the circulation were quantitated with a cell life-span concept. Drug effects were assumed to take place in the bone marrow based on irreversible linear or capacity-limited cytotoxicity. Mathematical derivations and computer simulations (Adapt II) were used to examine the properties of the model. Data from the literature were also analyzed. Cell response profiles after therapy typically exhibit a lag period, reduction to a nadir, and return to baseline. The predicted values of the time periods of granulopoiesis were 10–14 days for proliferation, and 1–6 days for maturation of progenitor cells in the bone marrow. The proposed irreversible mechanism of cell killing by anticancer drugs explains previously observed relationships between leukocyte nadir counts and exposure to the drug and/or duration of drug concentrations above some threshold level. The model was applied to literature data for paclitaxel and etoposide effects on leukocyte counts. The predicted value of KC50 for paclitaxel ranged from 0.004 to 0.2 μg/mL and for etoposide 2 μg/mL. The present model accounts for drug-induced leukopenia using a physiologic cell production and loss model and irreversible cytotoxicity in a precursor pool.
Similar content being viewed by others
REFERENCES
C. F. Stewart, S. G. Arbuck, R_ A. Fleming, and W. E. Evans. Relation of systemic exposure to unbound etoposide and hematologic toxicity. Clin. Pharmacol. Ther. 50:385–393 (1991).
T. Ohtsu, Y. Sasaki, T. Tamura, Y. Miyata, H. Nakanomyo, Y. Nishiwaki, and N. Saijo. Clinical pharmacokinetics and pharmacodynamics of paclitaxel: a 3–hour infusion vs. a 24–hour infusion. Clin. Cancer Res. 1:599–606(1995).
L. Gianni, C. M. Kearns, A. Giani, G. Capri, L. Viganó, A. Locatelli, G. Bonadonna, and M. Egorin. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic /pharmacodynamic relationship in humans. J. Clin. Oncol. 13:180–190 (1995).
M. O. Karlsson, R. E. Port, M. R. Ratain, and L. B. Sheiner. A population model for the leukopenic effect of etoposide. Clin. Pharmacol. Ther. 57:325–334 (1995).
H. Minami, Y. Sasaki, N. Saijo, T. Ohtsu, H. Fujii, T. Igarashi, and K. Itoh. Indirectresponse model for the time course of leukopenia with anticancer drugs. Clin. Pharmacol. Ther. 64:511–521 (1998).
M. O. Karlsson, V. Molnar, J. Bergh, A. Freijs, and R. Larson. A general model for timedissociated pharmacokinetic-pharmacodynamic relationships exemplified by paclitaxel myelosuppression. Clin. Pharmacol. Ther. 63:11–25 (1998).
A. S. Fokas, J. B. Keller, and B. D. Clarkson. Mathematical model of granulopoiesis and chronic myelogenous leukemia. Cancer Res. 51:2084–2091 (1991).
N. L. Dayneka, V. Garg, and W. J. Jusko. Comparison of four basic models of indirect pharmacodynamic responses. J. Pharmacokinet. Biopharm. 21:457–478 (1993).
W. J. Jusko. Pharmacodynamics of chemotherapeutic effects: dose-time-response relationships for phase-nonspecific agents J. Pharm. Sci. 60:892–895 (1971).
W. Krzyzanski, R. Ramakrishnan, and W. J. Jusko. Basic pharmacodynamic models for agents which alter production of natural cells. J. Pharmacokinet. Biopharm. 27:467–489 (1999).
N. M. Blackett and K. Adams. Cell proliferation and the action of cytotoxic agents on haemopoietic tissue. Br. J. Haematol. 23:751–758 (1972).
I. Boll and A. Ku¨hn. Granulocytopoiesis in human bone marrow cultures studied by means of kinematography. Blood 26:449–470 (1965).
E. Gunsilius, G. Gastl, and A. L. Petzer. Hematopoietic stem cells. Biomed. Pharmacother. 55:186–194 (2001).
W. J. Jusko, A pharmacodynamic model for cell-cycle-specific chemotherapeutic agents. J. Pharmacokin. Biopharm. 1:175–201 (1973).
W. Krzyzanski. Asymptotics of the total net direct pharmacological effect for large doses. J. Math. Biol. 41:477–492 (2000).
S. B. McKenzie. Textbook of Hematology, 2nd. ed., Williams & Wilkins, Baltimore, 1996.
D. Z. D'Argenio and A. Schumitzky. A program package for simulation and parameter estimation in pharmacokinetics. Comput. Prog. Biomed. 1:115–194 (1979).
L. Schacter. Etoposide phosphate: what, why, where, and how? Seminars in Oncology 23:1–7 (1996).
E. M. Newman, J. H. Doroshow, S. J. Forman, and K. G. Blume. Pharmacokinetics of high-dose etoposide. Clin. Pharmacol. Ther. 43:561–564 (1988).
J. Zhi, C. H. Nightingale, and R. Quintiliani. Microbial pharmacodynamics of piperacillin in neutropenic mice of systemic infection due to Pseudomonas aeruginosa. J. Pharmacokin. Biopharm. 16:355–375 (1988).
F. Recchia, S. De Filippis, P. Torchio, S. Rea, A. Gulino, D. Quaglino, and L. Frati, Randomized trial of filgrastim vs. sequential filgrastim and molgramostim after dose-intensified carboplatin, cyclophosphamide, and etoposide. Am. J. Clin. Oncol. 20:209–214 (1997).
L. E. Friberg, A. Freijs, M. Sandström, and M. O. Karlsson. Semiphysiological model for the time course of leukocytes after varying schedules of 5–fluorouracil in rats. J. Pharmacol. Exp. Ther. 295:734–740 (2000).
M. Katashima, Y. Yamada, K. Yamamoto, H. Kotaki, H. Sato, Y. Sawada, and T. Iga. Analysis of antiplatelet effect of ticlopidine in humans: modeling based on irreversible inhibition of platelet precursors in bone marrow. J. Pharmacokinet. Biopharm. 27:283–296 (1999).
L. G. Lajtha, R. Oliver, and C. W. Gurney. Kinetic model of a bone-marrow stem-cell population. Brit. J. Haemat. 8:442–460 (1962).
W. J. Jusko. Interpretation of cell proliferation curves using a two-compartment cell model. Math. Biosci. 21:31–37 (1974).
T. D. Jones, M. D. Morris, R. W. Young, and R. A. Kehlet. A cell-kinetics model for radiation-induced myelopoiesis. Exp. Hematol. 21:816–822 (1993).
G. J. Fetterly, J. Tamburlin, and R. Straubinger. Paclitaxel pharmacodynamics: Application of a mechanism-based neutropenia model. Biopharm.& Drugs Disp. 22:251–261 (2001).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krzyzanski, W., Jusko, W.J. Multiple-Pool Cell Lifespan Model of Hematologic Effects of Anticancer Agents. J Pharmacokinet Pharmacodyn 29, 311–337 (2002). https://doi.org/10.1023/A:1020984823092
Issue Date:
DOI: https://doi.org/10.1023/A:1020984823092