Abstract
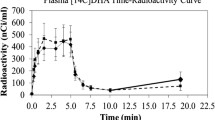
The Fatty Acid method was used to determine whether incorporation of plasma radiolabeled arachidonic acid into brain phospholipids is controlled by phospholipase A2. Awake rats received an i.v. injection of a phospholipase A2 inhibitor, manoalide (10 mg/kg), and then were infused i.v. with [1-14C]arachidonate or [3H]arachidonate. Animals were killed after infusion by microwave irradiation, and tracer distribution was analyzed in brain phospholipid, neutral lipid and acyl-CoA pools. Calcium-independent phospholipase A2 activity in brain homogenate was reduced by manoalide, whereas phospholipase C activity was unaffected. At 60 min but not at 20 or 40 min after its injection, manoalide had significantly decreased by 50% incorporation of unesterified arachidonate into and turnover within brain phospholipids, taking into account dilution of the brain arachidonoyl-CoA pool by recycled arachidonate. Manoalide also increased by 100% the net rate of unesterified arachidonate incorporation into brain triacylglycerol. This study indicates that manoalide can be used to inhibit brain phospholipase A2 in vivo, and that phospholipase A2 plays a critical role in arachidonate turnover in brain phospholipids and neutral lipids.
Similar content being viewed by others
REFERENCES
Irvine, R. F. 1982. How is the level of free arachidonic acid controlled in mammalian cell? Biochem J. 204:3-16.
Needleman, P., Turk, J., Jakschik, B. A., Morrisson, A. R., and Lefkowith, J. B. 1986. Arachidonic acid metabolism. Annu. Rev. Biochem. 55:69-102.
Sun, G.Y., and MacQuarrie, R. A. 1989. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann. NY Acad. Sci. 559:37-55.
Prescott, S. M., and Majerus, P. W. 1981. The fatty acid composition of phosphatidylinositol from thrombin-stimulated human platelets. J. Biol Chem. 756:579-582.
Washizaki, K., Smith, Q. R., Rapoport, S. I., and Purdon, A. D. 1994. Brain arachidonic acid incorporation and precursor pool specific activity during intravenous infusion of unesterified [3H]arachidonate in the anesthetized rat. J. Neurochem. 63:727-736.
Nariai, T., DeGeorge, J. J., Greig, N. H., Genka, S., Rapoport, S. I., and Purdon, A. D. 1994. Differences in rates of incorporation of intravenously injected radiolabeled fatty acids into phospholipids of intracerebrally implanted tumor and brain in awake rats. Clin. Exp. Metastasis 12:213-225.
Piomelli, D. 1993. Arachidonic acid in cell signaling. Curr. Opin. Cell Biol. 5:274-280.
Lombardo, D., and Dennis, E. A. 1985. Cobra venom phospholipase A2 inhibition by manoalide: A novel type of phospholipase inhibitor. J. Biol. Chem. 260:7234-7240.
Grange, E., Chang, M. C., Rabin, O., Bell, J., and Rapoport, S. I. 1995. Effect of a phospholipase A2 inhibitor on arachidonate incorporation into brain lipids in vivo. Trans. Am. Soc. Neurochem. 64:S 11.
Grange, E., Deutsch, J., Smith, Q. R., Chang, M. C., Rapoport, S. I., and Purdon, A. D. 1995. Specific activity of brain palmitoyl-CoA provides rates of incorporation of palmitate in brain phospholipids in awake rats. J. Neurochem. 65:2290-2298.
Folch, J., Lees, M., and Stanley, G. H. S. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509.
Skipski, V. P., Good, J. J., Barklay, M., and Reggio, R. B. 1968. Quantitative analysis of simple lipid classes by thin-layer chromatography. Biochim. Biophys. Acta 152:10-19.
Robinson P. J., Noronha J., DeGeorge, J. J., Freed, L. M., Nariai, T., and Rapoport, S. I. 1992. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res. Rev. 17:187-214.
Chang, M. C. J., Grange, E., Rabin, O., Bell, J. M., Allen, D. D., and Rapoport, S. I. 1996. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci. Lett. 220:171-174. Erratum 222:141.
Deutsch, J., Grange, E., Rapoport, S. I., and Purdon, A. D. 1994. Isolation and quantification of acyl-CoA esters in brain tissue by solid phase extraction. Anal. Biochem. 220:321-323.
Chang, M. C., Berkery, D., Laychock, S. G., and Schuel, H. 1991. Reduction of the fertilizing capacity of sea urchin sperm by cannabinoids derived from marihuana. III. Activation of phospholipase A2 in homogenate of sea urchin sperm by delta-9-tetrahydrocannabinol. Biochem. Pharmacol. 42:899-904.
Chang, M. C. J., and Jones, C. R. 1998. Chronic lithium treatment decreases brain phospholipase A2 activity. Neurochem. Res. 23:887-892.
Hirashima, Y., farooqui, A. A., Mills, J. S., and Horrocks, L. A. 1992. Identification and purification of calcium-independent phospholipase A2 from bovine brain cytosol. J. Neurochem. 59:708-714.
Yoshihara, Y., and Watabene, Y. 1990. Translocation of phospholipase A2 from cytosol to membranes in rat brain induced by calcium ions. Biochem. Biophys. Res. Commun. 170:484-490.
Waite, M. 1987. Handbook of Lipid Research: Vol. 5, The Phospholipases. Plenum Press, New York.
Perret, B., Chap, H. J., and Douste-Blazy, L. 1979. Asymmetric distribution of arachidonic acid in the plasma membrane of human platelets. A determination using purified phospholipases and a rapid method for membrane isolation. Biochim. Biophys. Acta. 556:434-446.
Clark, J. D., Lin, L.-L., Kriz, R. W., Ramesha, C. S., Sultzman, L. A., Lin, A. Y., Milona, N. and Knopf, J. L. 1991. A novel, arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell 65:1043-1051.
Fujimori, Y., Murakami, M., Kim, D. K., Hara, S., Takayama, K., Kudo, I., and Inoue, K. 1992. Immunochemical detection of arachidonoyl-preferential phospholipase A2. J. Biochem. 111:54-60.
Pierik, A. J., Nijssen, J. G., Aarsman, A. J., and van den Bosch, H. 1988. Calcium-independent phospholipase A2 in rat tissue cytosols. Biochem. Biophys. Acta 962:345-353.
Mayer, R. J., and Marshall, L. A. 1993. New insights on mammalian phospholipase A2(s); comparaison of arachidonoyl-selective and non selective enzymes. FASEB J. 7, 339-348.
Diaz-Arrastia, R., Ma L., and Jones S. 1996. Calcium-independent cytosolic phospholipase A2 (cicPLA2) is the major isoform in vertebrate brain and expressed in the limbic system. Trans. Am. Soc. Neurosci. 22 (1996) 620.
Stephenson, D. T., Manetta, J. V., White, D. L., Grace Chiou, X., Cox, L., Gitter, B., May, P. C., Sharp, J. D., Kramer R. M., and Clemens, J. A. 1994. Calcium-sensitive cytosolic phospholipase A2 (cPLA2) is expressed in human brain astrocytes. Brain Res. 637:97-105.
Bennett, C. F., Mong, S., Wu, H-L. W., Clarke, M. A., Wheeler, L., and Crooke, S. T. 1987. Inhibition of phosphoinoside-specific phospholipase C by manolaide. Mol Pharmacol. 32:587-593.
Hanel, A. M., Schuttel, S., and Gelb, M. H. 1993. Processive interfacial catalysis by mammalian 85-kDa phospholipase A2 enzymes on product-containing vesicles: application to the determination of substrate preferences. Biochemistry 32:5949-5958.
Bennett, C. F., Mong, S., Clarke, M. A., Kruse, L. I., and Crooke, S. T. 1987. Differential effects of manoalide on secreted and intracellular phospholipases. Biochem. Pharmacol 36:733-740.
Bianco, I. D., Kelley, M. J., Crowl, R. M., and Dennis, E. A. 1995. Identification of two specific lysines responsible for the inhibition of phospholipase A2 by manolaide. Biochim. Biophys. Acta 1250:197-203.
Yoshida, S., Ikeda, M., Busto, R., Santiso, M., Martinez, E., and Ginsberg, M. D. 1986. Cerebral phosphoinoside, triacylglycerol, and energy metabolism in reversible ischemia: origin and fate of free fatty acids. J. Neurochem. 47:744-757.
Waku, K. 1992. Origins and fates of fatty acyl-CoA esters. Biochim. Biophys. Acta 1124:101-111.
Chang, M. C. J., Grange, E., Rabin, O., and Bell, J. M., 1997. Incorporation of [U-14C]palmitate into rat brain: effect of an inhibitor of β-oxidation. J. Lipid Res. 38:295-300.
Sun, G. Y., and Su, K. L. 1979. Metabolism of arachidonoyl phosphoglycerides in mouse brain subcellular fractions. J. Neurochem. 32:1053-1959.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grange, E., Rabin, O., Bell, J. et al. Manoalide, a Phospholipase A2 Inhibitor, Inhibits Arachidonate Incorporation and Turnover in Brain Phospholipids of the Awake Rat. Neurochem Res 23, 1251–1257 (1998). https://doi.org/10.1023/A:1020788031720
Issue Date:
DOI: https://doi.org/10.1023/A:1020788031720