Abstract
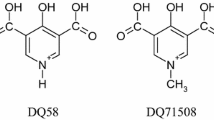
Purpose. Two dicarboxylate endogenous substances, bilirubin (BR) and 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF), have a very high affinity to human serum albumin (HSA). This study was undertaken to clarify the existence of a dicarboxylate binding site on HSA.
Methods. Chemical modification, pH dependent binding and X-ray crystallographic analysis were performed to characterize these dicarboxylate binding sites.
Results. It was found the binding behavior for dicarboxylates was different from typical site I ligands such as warfarin (WF) and phenyl-butazone (PB) and that electrostatic interaction was an important factor for their binding to HSA. Moreover, His residues were considered to play an important role in pH dependent binding of dicarboxylic acids but in a different manner from the site I ligands. X-ray crystallography of CMPF and BR revealed the distances between the two carboxyl groups in their chemical structures were 5.854 Å and 9.979 Å, respectively. This difference may be reflected in pH dependent binding. Using fluorescent probe displacement, we attempted to identify the binding site for monocarboxylate derivatives of CMPF and investigated the role of individual carboxyl group in the recognition of the binding site. The results suggested two carboxyl groups were important for the specific binding of CMPF to site I.
Conclusions. The binding site for dicarboxylic acids is located in subdomain IIA, which includes site I, on the HSA molecule. Electrostatic interaction is an important driving force for binding to HSA.
Similar content being viewed by others
REFERENCES
H. Mabuchi and H. Nakahashi. Isolation and characterization of an endogenous drug-binding inhibitor present in uremic serum. Nephron. 44:277–81 (1986).
H. Mabuchi, H. Nakahashi, T. Hamajima, I. Aikawa, and T. Oka. The effect of renal transplantation on a major endogenous ligand retained in uremic serum. Am. J. Kidney Dis. 13:49–54 (1989).
T. Sakai, A. Takadate, and M. Otagiri. Characterization of binding site of uremic toxins on human serum albumin. Biol. Pharm. Bull. 18:1755–61 (1995).
J. J. McTigue, S. J. Henderson, and W. E. Lindup, Excretion of the uraemic metabolite 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid in human urine. Nephron. 55:214–5 (1990).
M. G. Costigan, C. A. Callaghan, and W. E. Lindup. Hypothesis: is accumulation of a furan dicarboxylic acid (3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid) related to the neurological abnormalities in patients with renal failure? Nephron. 73:169–73 (1996).
C-F. Lim, R. Docter, E. P. Krenning, H. van Toor, B. Bernard, M. de Jong, and G. Hennemann. Transport of thyroxine into cultured hepatocytes: effects of mild non-thyroidal illness and calorie restriction in obese subjects. Clin. Endocrinol. 40:79–85 (1994).
S. J. Henderson and W. E. Lindup. Renal organic acid transport: uptake by rat kidney slices of a furan dicarboxylic acid which inhibits plasma protein binding of acidic ligands in uremia. J. Pharmacol. Exp. Ther. 263:54–60 (1992).
R. Brodersen. Bilirubin. Solubility and interaction with albumin and phospholipid. J. Biol. Chem. 254:2364–2369 (1979).
K. J. Fehske, W. E. Muller, and U. Wollert. The location of drug binding sites in human serum albumin. Biochem. Pharmacol. 30:687–692 (1981).
J. M. B. Octaaf, L. M. V. Eugene, C. I. B. Jan Paul, J. E. F. Marcel, W. Jaap, and H. M. J. Lambert. Location and characterization of the suramin binding sites of human serum albumin. Biochem. Pharmacol. 40:1595–1599 (1990).
R. F. Chen. Removal of fatty acids from serum albuminby charcoal treatment. J. Biol. Chem. 242:173–181 (1967).
M. G. Costigan, T. L. Gilchrist, and W. E. Lindup. Synthesis and physicochemical properties of the furan dicarboxylic acid, 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid, an inhibitor of plasma protein binding in uraemia. J. Pharm. Pharmacol. 48:635–40 (1996).
J. Pfordt, H. Thoma, and G. Spiteller. Identification, structure elucidation, and synthesis of previously unknown urofuranic acids in human blood. Liebigs Ann. Chem. 2298–2308 (1981).
R. Brodersen. Competitive binding of bilirubin and drugs to human serum albumin studied by enzymatic oxidation. J. Clin. Invest. 54:1353–64 (1974).
R. L. Levine. Fluorescence-quenching studies of the binding of bilirubin to albumin. Clin. Chem. 23:2292–301 (1977).
G. Sudlow, D. J. Birkett, and D. N. Wade. The characterization of two specific drug binding sites on human serum albumin. Mol. Pharmacol. 11:824–32 (1975).
K. J. Fehske, W. E. Muller, and U. Wollert. The modification of the lone tryptophan residue in human serum albumin by 2-hydroxy-5-nitrobenzyl bromide. Characterization of the modified protein and the binding of L-tryptophan and benzodiazepines to the tryptophan-modified albumin. Hoppe Seylers Zeitschrift fur Physiologische Chemie. 359:709–17 (1978).
K. J. Fehske, W. E. Muller, and U. Wollert. A highly reactive tyrosine residue as part of the indole and benzodiazepine binding site of human serum albumin. Biochim. Biophys. Acta 577:346–59 (1979).
A. D. Gounaris and G. E. Perlmann. Succinylation of pepsinogen. J. Biol. Chem. 242:2739–45 (1967).
R. Haynes, D. T. Osuga, and R. E. Feeney. Modification of amino groups in inhibitors of proteolytic enzymes. Biochemistry 6:541–7 (1967).
J. L. Roosemont. Reaction of histidine residues in proteins with diethylpyrocarbonate: differential molar absorptivities and reactivities. Anal. Biochem. 88:314–20 (1978).
H. Aki and M. Yamamoto. Thermodynamic characterization of drug binding to human serum albumin by isothermal titration microcalorimetry. J. Pharm. Sci. 83:1712–6 (1994).
C. B. Berde, B. S. Hudson, R. D. Simoni, and L. A. Sklar. Human serum albumin. Spectroscopic studies of binding and proximity relationships for fatty acids and bilirubin. J. Biol. Chem. 254:391–400 (1979).
D. A. Lightner, D. L. Holmes, and A. F. McDonagh. On the acid dissociation constants of bilirubin and biliverdin. pKa values from 13C NMR spectroscopy. J. Biol. Chem. 271:2397–405 (1996).
C. Jacobsen. Lysine residue 240 of human serum albumin is involved in high-affinity binding of bilirubin. Biochem. J. 171:453–459 (1978).
S. Curry, H. Mandelkow, P. Brick, and N. Franks. Crystal structure of human serum albumin complexed with fatty acid reveals an asymmetric distribution of binding sites. Nature Structural Biology 5:827–835 (1998).
N. Roosdorp, B. Wann, and I. Sjoholm. Correlation between arginyl residue modification and benzodiazepine binding to human serum albumin. J. Biol. Chem. 252:3876–3880 (1977).
I. Sjoholm, B. Ekman, A. Kober, I. Ljungstedt-Pahlman, B. Seiving, and T. Sjodin. Binding of drug to human serum albumin: XI. The specificity of three binding sites as studied with albumin immobilized in microparticles. Mol. Pharmacol. 16:767–777 (1979).
J. Jacobsen and R. Brodersen. Albumin-bilirubin binding mechanism. J. Biol. Chem. 258:6319–26 (1983).
J. H. Tonsgard and S. C. Meredith. Characterization of the binding sites for dicarboxylic acids on bovine serum albumin. Biochem. J. 276:569–575 (1991).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsutsumi, Y., Maruyama, T., Takadate, A. et al. Interaction Between Two Dicarboxylate Endogenous Substances, Bilirubin and an Uremic Toxin, 3-Carboxy-4-Methyl-5-Propyl-2-Furanpropanoic Acid, on Human Serum Albumin. Pharm Res 16, 916–923 (1999). https://doi.org/10.1023/A:1018842506896
Issue Date:
DOI: https://doi.org/10.1023/A:1018842506896