Abstract
Recent advances in the field of carrier-mediated intestinal absorption of of amino acids, oligopeptides, monosaccharides, monocarboxylic acids, phosphate, bile acids and several water-soluble vitamins across brush-border and basolateral membranes are summarized. An understanding of the molecular and functional characteristics of the intestinal membrane transporters will be helpful in the utilization of these transporters for the enhanced oral delivery of poorly absorbed drugs. Some successful examples of the synthesis of prodrugs recognized by the targeted transporters are described. Functional expression of the multidrug resistance gene product, P-glycoprotein, as a primary active transporter in the intestinal brush-border membrane leads to net secretion of some drugs such as anticancer agents in the blood-to-luminal direction, serving as a secretory detoxifying mechanism and as a part of the absorption barrier in the intestine.
Similar content being viewed by others
REFERENCES
T. Hoshi. Proton-coupled transport of organic solutes in animal cell membranes and its relation to Na+ transport. Japn. J. Phys. 35:179–191 (1985).
M. Lucus. Determination of acid surface pH in vivo in rat proximal jejunum. Gut 24:734–739 (1983).
H. Murer, U. Hopfer, and R. Kinne. Sodium/proton antiport in brush-border membrane vesicles isolated from rat small intestine and kidney. Biochem. J. 154:597–604 (1976).
V. Ganapathy, M. Brandsch, and F. H. Leibach. Intestinal transport of amino acids and peptides. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract, third ed., Raven Press, New York, N. Y., pp.1773–1794 (1994).
H. N. Christensen. Distinguishing amino acid transport systems of a given cell or tissue. Meth. Enzymol. 173:576–616 (1989).
L. K. Munck and B. G. Munck. Chloride-dependence of amino acid transport in rabbit ileum. Biochim. Biophys. Acta 1027:17–20 (1990).
Y. Miyamoto, C. Tirrupathi, V. Ganapathy, and F. H. Leibach. Active transport of taurine in rabbit jejunal brush-border membrane vesicles. Am. J. Physiol. 257:G65–G72 (1989).
D. T. Thwaites, G. T. A. McEwan, B. H. Hirst, and N. L. Simmons. H+-coupled α-methylaminoisobutyric acid transport in human intestinal Caco-2 cells. Biochim. Biophys. Acta 1234:111–118 (1995).
M. A. Hediger, Y. Kanai, G. You, and S. Nussberger. Mammalian ion-coupled solute transporters. J. Physiol. 482, 7S–17S (1995).
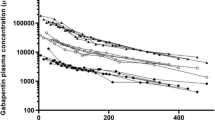
B. H. Stewart, A. R. Kugler, P. R. Thompson, and N. Bockbrader. A saturable transport mechanism in the intestinal absorption of gabapentin is the underlying cause of the lack of proportionality between increasing dose and drug levels in plasma. Pharm. Res. 10:276–281 (1993).
G. L. Amidon, A. E. Merfeld, and J. B. Dressman. Concentration and pH dependency of α-methyldopa absorption in rat intestine. J. Pharm. Pharmacol. 38:363–368 (1986).
M. Hu and R. T. Borchardt. Mechanism of L-α-methyldopa transport through a monolayer of polarized human intestinal epithelial cells (Caco-2). Pharm. Res. 7:1313–1319 (1990).
H. Shindo, T. Komai, and K. Kawai. Studies on the metabolism of D-and L-isomers of 3,4-dihydroxyphenylalanine (DOPA). V. Mechanism of intestinal absorption of D-and L-DOPA-14C in rats. Chem. Pharm. Bull (Tokyo). 21:2031–2038 (1973).
T. Cercos-Fortea, A. Polache, A. Nacher, E. Cejudo-Ferragud, V. G. Casabo, and M. Merino. Influence of leucine on intestinal baclofen absorption as a model compound of neutral α-amino acids. Biopharm. Drug Dispos. 16:563–577 (1995).
D. T. Thwaites, G. Armstrong, B. H. Hirst, and N. L. Simmons. D-cycloserine transport in human intestinal epithelial (Caco-2) cells mediated by a H+-coupled amino acid transporter. Brit. J. Pharmacol. 115:761–766 (1995).
V. Ganapathy and F. H. Leibach. Is intestinal peptide transport energized by a proton gradient? Am. J. Physiol. 249:G153–G160 (1985).
H. Minami, E. L. Morse, and S. A. Adibi. Characteristics and mechanism of glutamine-dipeptide absorption in human intestine. Gastroenterology 103:3–11 (1992).
Y.-J. Fei, Y. Kanai, S. Nussberger, V. Ganapathy, F. H. Leibach, M. F. Romero, S. K. Singh, W. F. Boron, and M. A. Hediger. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature 368:563–566 (1994).
R. Liang, Y.-J. Fei, P. D. Presad, S. Ramamoorthy, H. Han, T. L. Yang-Feng, M. A. Hediger, V. Ganapathy, and F. H. Leibach. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J. Biol. Chem. 270:6456–6463 (1995).
K. Miyamoto, T. Shiraga, H. Yamamoto, H. Haga, Y. Taketani, K. Morita, I. Tamai, Y. Sai, A. Tsuji, and E. Takeda. Sequence, tissue distribution and developmental changes in rat intestinal oligopeptide transporter. Biochem. Biophys. Acta, 1305:34–38(1996).
I. Tamai, K. Hayashi, T. Terao, Y. Sai, T. Shiraga, K. Miyamoto, E. Takeda, H. Higashida, and A. Tsuji. H+ coupled transport of β-lactam antibiotics mediated by oligopeptide transporter, PepT1, cloned from rat small intestine. submitted.
J. Dyer, R. B. Beechey, J.-P. Gorvel, R. T. Smith, R. Wootton, and S. P. Shirazi-Beechey. Glycyl-L-proline transport in rabbit enterocyte basolateral membrane vesicles. Biochem. J. 269:565–571 (1990).
D. T. Thwaites, C. D. A. Brown, B. H. Hirst, and N. L. Simmons. Transepithelial glycylsarcosine transport in intestinal Caco-2 cells mediated by expression of H+-coupled carriers at both apical and basal membranes. J. Biol. Chem. 268:7640–7642 (1993).
W. Liu, R. Liang, S. Ramamoorthy, Y-J. Fei, M. E. Ganapathy, M. A. Hediger, V. Ganapathy, and F. H. Leibach. Molecular cloning of PEPT 2, a new member of the H+/peptide cotransport family, from human kidney. Biochim. Biophys. Acta 1235:461–466 (1995).
A. Tsuji. Intestinal absorption of β-lactam antibiotics; In M. D. Taylor and G. L. Amidon (eds.), Peptide-based drug design, American Chemical Society, Washington, DC, pp. 299–316 (1995).
T. Okano, K. Inui, H. Maegawa, M. Takano, and R. Hori. H+ copuled uphill transport of aminocephalosporins via the dipeptide transport system in rabbit intestinal brush-border membranes. J. Biol. Chem. 261:14130–14134 (1986).
K. Inui, M. Miyamoto, and H. Saito. Transepithelial transport of oral cephalosporins by monolayers of intestinal epithelial cell line Caco-2: Specific transport systems in apical and basolateral membranes. J. Pharmacol. Exp. Ther. 261:195–201 (1992).
I. Tamai, N. Tomizawa, A. Kadowaki, T. Terasaki, K. Nakayama, H. Higashida, and A. Tsuji. Functional expression of intestinal depeptide/β-lactam antibiotic transporter in Xenopus laevis oocytes. Biochem. Pharmacol. 48:881–888 (1994).
I. Tamai, N. Tomizawa, T. Takeuchi, K. Nakayama, H. Higashida, and A. Tsuji. Functional expression of transporter for β-lactam antibiotics and dipeptides in Xenopus laevis oocytes injected with messenger RNA from human, rat and rabbit small intestines. J. Pharmacol. Exp. Ther. 273:26–31 (1995).
M. Boll, D. Markovich, W.-M. Weber, H. Korter, H. Daniel, and H. Murer. Expression cloning of a cDNA from rabbit small intestine related to proton-coupled transport of peptides, β-lactam antibiotics and ACE-inhibitors. Pflugers-Arch. 429:146–149 (1994).
G. L. Amidon and H. J. Lee. Absorption of peptide and peptidomimetic drugs. Annu. Rev. Pharmacol. Toxicol. 34:321–341 (1994).
S. Yee and G. L. Amidon. Oral absorption of angiotensin-converting enzyme inhibitors and peptide prodrugs; In M. D. Taylor and G. L. Amidon (eds.), Peptide-based drug design, American Chemical Society, Washington, DC, pp. 299–316. (1995).
W. Kramer, F. Girbig, U. Gutjahr, H. W. Kleemann, I. Leipe, H. Urbach, and A. Wagner. Interaction of renin inhibitors with the intestinal uptake system for oligopeptides and β-lactam antibiotics. Biochim. Biophys. Acta 1027:25–30 (1990).
N. Hashimoto, T. Fujioka, T. Toyoda, N. Muranishi, and K. Hirano. Renin inhibitor: transport mechanism in rat small intestinal brush-border membrane vesicles. Pharm. Res. 11:1448–1451 (1994).
M. Takano, Y. Tomita, T. Katsura, M. Yasuhara, K. Inui, and R. Hori. Bestatine trasnport in rabbit intestinal brush-border membrane vesicles. Biochem. Pharmacol. 47:1089–1090 (1994).
E. Walter, T. Kissel, M. Reers, G. Dickneite, D. Hofmann, and W. Stuber. Transepithelial properties of peptidomimetic thrombin inhibitors in monolayers of a human intestinal cell line (Caco-2) and their correlation to in vivo data. Pharm. Res. 12:360–365 (1995).
A. Tsuji, I. Tamai, M. Nakanishi, and G. L. Amidon, Mechanism of absorption of the dipeptide α-methyldopa-phe in the intestinal brush-border membrane vesicles. Pharm. Res. 7:308–309 (1990).
J. P. F. Bai. pGlu-L-dopa-pro: A tripeptide prodrug targeting the intestinal peptide transporter for absorption and tissue enzymes for conversion. Pharm. Res. 12:1101–1104 (1995).
J. R. Pappenheimer, and J. M. Madara. Role of active transport in regulation of junctional permeability and paracellular absorption of nutrients by intestinal epithelia. In H. Ussing (ed.), Transport in leaky epithelial, Copenhagen, Munksgaard (1993).
M. A. Hediger, M. J. Coady, T. S. Ikeda, and E. M. Wright. Expression cloning and cDNA sequencing of the Na+/glucose cotransporte. Nature 330:379–381 (1987).
M. A. Hediger and D. B. Rhoads. Molecular physiology of sodium-glucose cotransporters. Physiol. Rev. 74:993–1026 (1994).
W.-S. Lee, Y. Kanai, R. G. Wells, and M. A. Hediger. The high affinity Na+/glucose cotransporter. Re-evaluation of function and distribution of expression. J. Biol. Chem. 269:12032–12039 (1994).
T. Kayano, C. F. Burant, H. Fukumoto, G. W. Gould, Y-S. Fan,, R. L. Eddy, M. G. Byers, T. B. Shoes, S. Seino, and G. I. Bell. Human facilitative glucose transporters. J. Biol. Chem. 265:13276–13282 (1990).
E. B. Rand, A. M. Depaoli, N. O. Davidson, G. I. Bell, and C. F. Burant. Sequence, tissue distribution, and functional characterization of the rat fructose transporter GLUT5. Am. J. Physiol. 264:G1169–1176 (1993).
K. Miyamoto, S. Tatsumi, A. Morimoto, H. Minami, H. Yamamoto, K. Sone, Y. Taketani, Y. Nakabou, T. Oka, and E. Takeda. Characterizastion of the rabbit intestinal fructose transporter (GLUT5). Biochem. J. 303:877–883 (1994).
H. Fukumoto, S. Seino, H. Imura, Y. Seino, R. L. Eddy, Y. Fukushima, M. G. Byers, T. B. Shows, and G. I. Bell. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc. Natl. Acad. Sci. USA 85:5434–5438 (1988).
G. W. Gould, H. M. Thomas, T. J. Jess, and G. I. Bell. Expression of human glucose transporters in Xenopus Oocytes: kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry 30:5139–5145 (1991).
B. Thorens, Z-Q. Cheng, D. Brown, and H. F. Lodish. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am. J. Physiol. 259:C279–285 (1990).
T. Mizuma, K. Ohta, M. Hayashi, and S. Awazu. Intestinal active absorption of sugar-conjugated compounds by glucose transport system: implication of improvement of poorly absorbable drugs. Biochem. Pharmacol. 43:2037–2039 (1992).
T. Mizuma, K. Ohta, and S. Awazu. The β-anomeric and glucose perferences of glucose transport carrier for intestinal active absorption of monosaccharide conjugates. Biochim. Biophys. Acta 1200:117–122 (1994).
T. Mizuma, N. Sakai, and S. Awazu. Na+-Dependent transport of aminopeptidase-resistant sugar-coupled tripeptides in rat intestine. Biochem. Biophys. Res. Commun. 203:1412–1416 (1994).
M., Haga, K. Saito, T. Shimaya, Y. Maezawa, Y. Kato, and S. W. Kim. Hypoglycemic effect of intestinally administered monosaccharide-modified insulin derivatives in rats. Chem. Pharm. Bull. 38:1983–1986 (1990).
G. Rechkemmer. Transport of weak electrolytes. In V. Schultz, Handbook of Physiology, American Physiological Society, Bethesda, MD, pp. 371–388 (1991).
M. L. Hougerle and D. Winne. Drug absorption by the rat jejunum perfused in situ. Dissociation from the pH-partition theory and role of microclimate-pH and unstirred layer. Naunyn-Schmiedebergs Arch Pharmacol. 322:249–255 (1983).
C. Tiruppathi, D. F. Balkovetz, V. Ganapathy, Y. Miyamoto, and F. H. Leibach. A proton gradient, not a sodium gradient, is the driving force for active transport of lactate in rabbit intestinal brush-border membrane vesicles. Biochem. J. 256:219–223 (1988).
M. Bugaut. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp. Biochem. Physiol. 86B:439–472 (1987).
A. Tsuji, M. T. Simanjuntak, I. Tamai, and T. Terasaki. pH-Dependent intestinal transport of monocarboxylic acids: carriermediated and H+-cotransport mechanism versus pH-partition hypothesis. J. Pharm. Sci. 79:1123–1124 (1990).
M. Dohgen, H. Hayashi, T. Yajima, and T. Suzuki. Stimulation of bicarbonate secretion by luminal short-chain fatty acids in the rat and human colon in vivo. Japn. J. Physiol. 44:519–531 (1994).
E. Titus and G. A. Aheam. Transintestinal acetate transport in a herbivorous teleost: anion exchange at the basolateral membrane. J. Exp. Biol. 156:41–61 (1991).
M. T. Simanjuntak, T. Terasaki, I. Tamai, and A. Tsuji. Participation of monocarboxylic anion and bicarbonate exchange system for the transport of acetic acid and monocarboxylic acid drugs in the small intestinal brush-border membrane vesicles. J. Pharmacobio-Dyn. 14:501–508 (1991).
J. M. Harig, K. H. Soergel, J. A. Barry, and K. Ramaswamy. Transport of propionate by ileal brush-border membrane vesicles. Am. J. Physiol. 260:G776–782 (1991).
R. C. Poole and A. P. Halestrap. N-Terminal protein sequence analysis of the rabbit erythrocyte lactate transporter suggests identity with the cloned mono-carboxylate transport protein MCT1. Biochem. J. 303:755–759 (1994).
C-K. Garcia, J. L. Goldstein, R. K. Pathak, R. G. W. Anderson, and M. S. Brown. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the cori cycle. Cell 76:865–873 (1994).
I. Tamai, H. Takanaga, H. Maeda, Y. Sai, T. Ogihara, H. Higashida, and A. Tsuji. Participation of a proton-cotransporter, MCT1 in the intestinal transport mechanism for monocarboxylic acids. Biochem. Biophys. Res. Commun. 214:482–489 (1995).
H. Takanaga, I. Tamai, S. Inaba, Y. Sai, H. Higashida, H. Yamamoto, and A. Tsuji. cDNA cloning and functional characterization of rat intestinal monocarboxylate transporter. Biochem. Biophys. Res. Commun. 217:370–377 (1995).
I. Osiecka, P. A. Porter, R. T. Borchardt, J. A. Fix, and C. R. Gardner. In vitro drug absorption models. I. Brush border membrane vesicles, isolated mucosal cells and everted intestinal rings: Characterization and salicylate accumulation. Pharm. Res. 2:284–293 (1985).
H. Takanaga, I. Tamai, and A. Tsuji. pH-Dependent and carrier-mediated transport of salicylic acid across Caco-2 cells. J. Pharm. Pharmacol. 46:567–570 (1994).
A. Tsuji, H. Takanaga, I. Tamai, and T. Terasaki. Transcellular transport of benzoic acid across Caco-2 cells by a pH-dependent and carrier-mediated transport mechanism, Pharm. Res. 11:30–37 (1994).
I. Tamai, H. Takanaga, H. Maeda, T. Ogihara, M. Yoneda, and A. Tsuji. Proton-cotransport of pravastatin across intestinal brush-border membrane. Pharm. Res. 12:1727–1732 (1995).
W. Berner, R. Kinne, and H. Murer. Phosphate transport into brush-border membrane vesicles isolated from rat small intestine. Biochem. J. 160:467–474 (1976).
S. M. Borowitz and F. K. Ghishan. Phosphate transport in human jejunal brush-border membrane vesicles. Gastroenterology 96:4–10 (1989).
A. Tsuji and I. Tamai. Na+ and pH dependent transport of foscarnet via the phosphate carrier system across intestinal brush-border membrane. Biochem. Pharmacol. 38:1019–1022 (1989).
P. W. Swaan and J. J. Tukker. Carrier-mediated transport mechanism of foscarnet (trisodium phosphonoformate hexahydrate) in rat intestinal tissue. J. Pharmacol. Exp. Ther. 272:242–247 (1994).
T. Ishizawa, A. Tsuji, I. Tamai, T. Terasaki, K. Hosoi, and S. Fukatsu. Sodium and pH dependent carrier-mediated transport of antibiotic, fosfomycin, in the rat intestinal brush-border membrane. J. Pharmacobio-Dyn. 13:292–300 (1990).
T. Ishizawa, S. Sadahiro, K. Hosoi, I. Tamai, T. Terasaki, and A. Tsuji. Mechanisms of intestinal absorption of the antibiotic, fosfomycin, in brush-border membrane vesicles in rabbits and humans. J. Pharmacobio-Dyn. 15:481–489 (1992).
T. Ishizawa, M. Hayashi, and S. Awazu. Effect of carrier-mediated transport system on intestinal fosfomysin absorption in situ and in vivo. J. Pharmacobio-Dyn. 14:82–86 (1991).
F. A. Wilson. Intestinal transport of bile acids. Am. J. Physiol. 241:G83–G92 (1981).
M. H. Wong, P. Oelkers, A. L. Craddock, and P. A. Dawson. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J. Biol. Chem. 269:1340–1347 (1994).
W. Kramer, G. Wess, G. Neckermann, G. Schubert, J. Fink, F. Girbig, U. Gutjahr, S. Kowalewski, K.-H. Baringhaus, G. Boger, A. Enhsen, E. Falk, M. Friedrich, H. Glombik, A. Hoffmann, C. Pittius, and M. Urmann, Intestinal absorption of peptides by coupling to bile acids. J. Biol. Chem. 269:10621–10627 (1994).
M. T. Simanjuntak, I. Tamai, T. Terasaki, and A. Tsuji. Carrier-mediated uptake of nicotinic acid by rat intestinal brush-border membrane vesicles and relation to monocarboxylic acid transport. J. Pharmacobio-Dyn. 13:301–309 (1990).
H. Takanaga, H. Maeda, I. Tamai, H. Higashida, and A. Tsuji. Nicotinic acid transport mediated by pH-dependent anion antiporter and proton cotransporter in rabbit intestinal brush-border membrane. J. Pharm. Pharmacol. in press.
R. C. Rose. Intestinal transport of water-soluble vitamins. In S. G. Schultz (ed.), Handbook of Physiology, American Physiological Society, Bethesda, MD, pp. 421–435 (1991).
H. M. Said, F. K. Ghishan, and R. Redha. Folate transport by intestinal brush-border membrane vesicles. Am. J. Physiol. 252:G229–G236 (1987).
H. M. Said and R. Redha. A carrier-mediated transport for folate in basolateral membrane vesicles of rat small intestine. Biochem. J. 247:141–146 (1987).
J. Zimmerman. Methotrexate transport in the human intestine. Biochem. Pharmacol. 43:2377–2383 (1992).
H. Matsue, K. G. Rothberg, A. Takashima, B. A. Kamen, R. G. W. Anderson, and S. W. Lacey. Folate receptor allows cells to grow in low concentrations of 5-methyltetrahydrofolate. Proc. Natl. Acad. Sci. USA 89:6006–6009 (1992).
H. Saitoh, M. Kobayashi, M. Sugawara, K. Iseki, and K. Miyazaki. Carrier-mediated transport system for choline and its related quaternary ammonium compounds on rat intestinal brush-border membrane. Biochim. Biophys. Acta 1112:153–160 (1992).
G. R. Herzberg and J. Lerner. Intestinal absorption of choline in the chick. Biochim. Biophys. Acta 307:234–242 (1972).
Z. C. Gatmaitan and I. M. Arias. Structure and function of P-glycoprotein in normal liver and small intestine. Adv. Pharmacol. 24:77–97 (1993).
D. Leveque and F. Jehl. P-glycoprotein and pharmacokinetics. Anticancer Res. 15:331–336 (1995).
A. H. Schinkel, J. J. M. Smit, O. van Tellingen, J. H. Beijnen, E. Wagnenaar, L. van Deemter, C. A. A. M. Mol, M. A. van der Valk, E. C. Robanus-Maandag, H. P. J. te Riele, A. J. M. Berns, and P. Borst. Distruction of the mouse mdrla P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 77:491–502 (1994).
A. Tsuji, T. Terasaki, Y. Takabatake, Y. Tenda, I. Tamai, T. Yamashita, S. Moritani, T. Tsuruo, and J. Yamashita. P-glycoprotein as drug efflux pump in primary cultured bovine brain capillary endothelial cells. Life Sci. 51:1427–1437 (1992).
A. Tsuji, I. Tamai, A. Sakata, Y. Tenda, and T. Terasaki. Restricted transport of cyclosporin A across the blood-brain barrier by a multidrug transporter, p-glycoprotein. Biochem. Pharmacol. 46:1096–1099 (1993).
A. Sakata, I. Tamai, K. Kawazu, Y. Deguchi, T. Ohnishi, A. Saheki, and A. Tsuji. In vivo evidence for ATP-dependent and P-glycoprotein-mediated transport of cyclosporin A at the blood-brain barrie. Biochem. Pharmacol. 48:1989–1992 (1994).
T. Ohnishi, I. Tamai, K. Sakanaka, A. Sakata, T. Yamashima, J. Yamashita, and A. Tsuji. In vivo and in vitro evidence for ATP-dependency of P-glycoprotein-mediated efflux of doxorubicin at the blood-brain barrier. Biochem. Pharmacol. 49:1541–1544 (1995).
J. Hunter, B. H. Hirst, and N. L. Simmons. Epithelial secretion of vinblastine by human intestinal adenocarcinoma cell (HCT-8 and T84) layers expressing P-glycoprotein. Br. J. Cancer 64:437–444 (1991).
M. B. Meyers, K. W. Scitto, and F. M. Sirotnak. P-Glycoprotein content and mediation of vincristine efflux: correlation with the level of differentiation in luminal epithelium of mouse small intestine. Cancer Commun. 3:159–165 (1991).
J. Hunter, B. H. Hirst, and N. L. Simmons. Drug absoprtion limited by P-glycoprotein-mediated secretory drug transport in human intestinal epithelial Caco-2 cell layers. Pharm. Res. 10:743–749 (1993).
B.-L. Leu and J.-D. Huang. Inhibition of P-glycoprotein and effects on etoposide absorption. Cancer Chemother. Pharmacol. 35:432–436 (1995).
V. Phung-Ba, A. Warnery, D. Schermann, and P. Wils. Interaction of pristinamycin IA with P-glycoprotein in human intestinal epithelial cells. Eur. J. Pharmacol. 288:187–192 (1995).
P. A. Augustijns, T. Timothy, P. Badshaw, L.-S. L. Gan, R. W. Hendren, and D. R. Thakker. Evidence for a polarized efflux system in Caco-2 cells capable of modulating cyclosporn A transport. Biochem. Biophys. Res. Commun. 197:360–365 (1993).
P. S. Burton, R. A. Conradi, A. R. Hilgers, and N. H. Ho. Evidence for a polarized efflux system for peptides in the apical membrane of Caco-2 cells. Biochem. Biophys. Res. Commun. 190:760–766 (1993).
J. Karlsson, S.-M. Kuo, J. Ziemniak, and P. Artursson. Transport of celiprolol across human epithelial (Caco-2) cells: mediation of secretion by multiple transporters including P-glycoprotein. Br. J. Pharmacol. 110:1009–1016 (1993).
H. Saitoh and B. J. Aungst. Possible involvement of multiple P-glycoprotein-mediated efflux system in the transport of verapamil and other organic cations across rat intestine. Pharm. Res. 12:1304–1310 (1995).
S. Hsing, Z. C. Gatmaitan, and I. M. Arias. The function of Gp170, the multidrug-resistance gene product, in the brush border of rat intestinal mucosa. Gastroenterology 102:879–885 (1992).
M. Naito, H. Tsuge, C. Kuroko, T. Koyama, A. Tomida, T. Tatsuya, Y. Heike, and T. Tsuruo. Enhancement of cellular accumulation of cyclosporine by anti-P-glycoprotein monoclonal antibody MRK-16 and synergistic modulation of multidrug resistance. J. Natl. Cancer Inst. 85:311–316 (1993).
I. Komiya, J. Y. Park, A. Kamani, N. F. H. Fo., and W. I. Higuchi. Quantitative mechanistic studies in simultaneous fluid flow and intestinal absorption using steroids as model solutes. Int. J. Pharm. 4:249–262 (1980).
Y. C. Martin. A practitioner's perspective of the role of quantitative structure-activity analysis in medicinal chemistry. J. Med. Chem. 24:229–237 (1981).
D. C. Taylor, R. Pownall, and W. Burke. The absorption of β-adrenoreceptor antagonists in rat in-situ small intestine; The effect of lipophilicity. J. Pharm. Pharmacol. 37:280–283 (1985).
S. J. Cirrier, K. Ueda, M. C. Willingham, I. Pastan, and M. Gottesman. Deletion and insertion mutant of the multidrug transporter. J. Biol. Chem. 264:14376–14381 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsuji, A., Tamai, I. Carrier-Mediated Intestinal Transport of Drugs. Pharm Res 13, 963–977 (1996). https://doi.org/10.1023/A:1016086003070
Issue Date:
DOI: https://doi.org/10.1023/A:1016086003070