Abstract
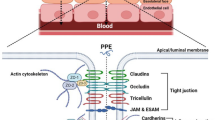
The brush border membrane of intestinal mucosal cells contains a peptide carrier system with rather broad substrate specificity and various endo- and exopeptidase activities. Small peptide (di-/ tripeptide)-type drugs with or without an N-terminal α-amino group, including β-lactam antibiotics and angiotensin-converting enzyme (ACE) inhibitors, are transported by the peptide transporter. Poly-peptide drugs are hydrolyzed by brush border membrane proteolytic enzymes to di-/tripeptides and amino acids. Therefore, while the intestinal brush border membrane has a carrier system facilitating the absorption of di-/tripeptide drugs, it is a major barrier limiting oral availability of polypeptide drugs. In this paper, the specificity of peptide transport and metabolism in the intestinal brush border membrane is reviewed.
Similar content being viewed by others
REFERENCES
S. A. Adibi and E. Phillips. Evidence for greater absorption of amino acid from peptide than from free form by human intestine. Clin. Res. 16:446–448 (1968).
D. M. Matthews and J. W. Payne. Transmembrane transport of small peptides. In Current Topics in Membrane and Transport, Academic Press, New York, 1980, Vol. 14, pp. 331–425.
D. I. Friedman and G. L. Amidon. Intestinal absorption mechanism of two prodrug ACE inhibitors in rats: Enalapril maleate and fosinopril sodium. Pharm. Res. 6:1043–1047 (1989).
D. I. Friedman and G. L. Amidon. The intestinal absorption mechanism of dipeptide ACE inhibitors of the lysyl-proline type: Lisinopril and SQ 29,852. J. Pharm. Sci. 78:995 (1989).
M. Hu and G. L. Amidon. Passive and carrier-mediated intestinal absorption components of captopril. J. Pharm. Sci. 77:1007–1011 (1988).
P. J. Sinko and G. L. Amidon. Characterization of the oral absorption of β-lactam antibiotics. I. Cephalosporins. Determination of intrinsic membrane absorption parameters in the rat intestine in situ. Pharm. Res. 5:645–650 (1988).
P. J. Sinko and G. L. Amidon. Characterization of the oral absorption of β-lactam antibiotics. II. Competitive absorption and peptide carrier specificity. J. Pharm. Sci. 78:723–726 (1989).
A. Tsuji, T. Terasaki, I. Tamai, and H. Hirooka. H+-gradient-dependent and carrier-mediated transport of cefixime, a new cephalosporin antibiotic, across brush-border membrane vesicles from rat small intestine. J. Pharmacol. Exp. Ther. 241:594–601 (1987).
S. A. Adibi and Y. S. Kim. Peptide absorption and hydrolysis. In L. R. Johnson (ed.), Physiology of the Gastrointestinal Tract, 1st ed., Raven Press, New York, 1981, p. 1073.
V. H. L. Lee, S. Dodda-Kashi, G. M. Grass, and W. Rubas. Oral route of peptide and protein drug delivery. In V. H. L. Lee (ed.), Peptide and Protein Drug Delivery, Marcel Dekker, New York, 1991, pp. 691–738.
M. Saffran, C. Bedra, G. S. Kumar, and D. C. Neckers. Vaspressin: A model for the study of effects of additives on the oral and rectal administration of peptide drugs. J. Pharm. Sci. 77:33–38 (1988).
K. Takaori, J. Burton, and M. Donawitz. The transport of an intact oligopeptide across adult mammalian jejunum. Biochem. Biophys. Res. Comm. 137:682–687 (1986).
A. J. Wood, G. Maurer, W. Niederberger, and T. Beveridge. Cyclosporine: Pharmacokinetics, metabolism, and drug interactions. Transplant. Proc. 15:2409 (1983).
M. Kidron, H. Bar-On, E. M. Berry, and E. Ziv. The absorption of insulin from various regions of the rat intestine. Life Sci. 31:2937–2841 (1982).
C. A. R. Boyd and M. R. Ward. A micro-electrode study of oligopeptide absorption by the small intestinal epithelium of Necturus maculosus. J. Physiol. 324:411–428 (1982).
J. M. Addison, D. Burston, J. W. Payne, S. Wilkison, and D. M. Matthews. Evidence for active transport of tripeptides by hamster jejunum in vitro. Clin. Sci. Mol. Med. 49:305–312 (1975).
W. Kramer, U. Gutjahr, F. Girbig, and I. Leipe. Intestinal absorption of dipeptides and β-lactam antibiotics. II. purification of the binding protein for dipeptides and β-lactam antibiotics from rabbit small intestinal brush border membrane. Biochim. Biophys. Acta 1030:50–55 (1990).
V. Ganapathy and F. H. Leibach. Is intestinal peptide transport energized by a proton gradient? Am. J. Physiol. 249:G153–G160 (1985).
J. M. Addison, D. M. Matthews, and D. Burston. Evidence for active transport of the dipeptide carnosine (β-alanyl-L-histidine) by hamster jejunum in vitro. Clin. Sci. Mol. Med. 46:707–714 (1974).
M. Das and A. N. Radhakrishnan. Studies on a wide-spectrum intestinal dipeptide uptake system in the monkey and in the human. Biochem. J. 146:133–139 (1975).
A. Rubino, M. Field, and H. Schwachman. Intestinal transport of amino acid residues of dipeptides. I. Influx of the glycine residue of glycyl-L-proline across mucosal border. J. Biol. Chem. 246:3542–3548 (1971).
J. M. Addison, D. Burston, J. A. Dalrymple, D. M. Matthews, J. W. Payne, M. H. Sleisenger, and S. Wilkinson. A common mechanism for transport of di-and tripeptides by hamster jejunum in vitro. Clin. Sci. Mol. Med. 49:313–322 (1975a).
Y. Tomita, T. Katsura, T. Okano, K. I. Inui, and R. Hori. Transport mechanisms of bestatin in rabbit intestinal brushborder membrane: Role of H+/dipeptide cotransport system. J. Pharmacol. Exp. Ther. 252:859–862 (1990).
P. F. Bai, P. Subramanian, H. I. Mosberg, and G. L. Amidon. Structural requirements for the intestinal mucosal cell peptide transporter: The need for N-terminal α-amino group. Pharm. Res. 8:593–599 (1991).
V. M. Rajendran, S. A. Ansari, J. M. Harig, M. B. Adams, A. H. Khan, and K. Ramaswamy. Transport of glycyl-L-proline by human intestinal brush border membrane vesicles. Gastroenterology 89:1298–1304 (1985).
A. M. Asatoor, A. Chadha, M. D. Milne, and D. I. Prosser. Intestinal absorption of stereoisomers of dipeptides in the rat. Clin. Sci. Mol. Med. 45:199–212 (1973).
C. I. Cheeseman and D. H. Smyth. Specific transfer process for intestinal absorption of peptides. Pro. Physiol. Soc. Nov:45p–46p (1972).
D. Burston, J. M. Addison, and D. M. Matthews. Uptake of dipeptides containing basic and acidic amino acids by rat small intestine in vitro. Clin. Sci. 43:823–837 (1972).
D. M. Oh, P. J. Sinko, and G. L. Amidon. Characterization of the oral absorption of some penicillins: Determination of intrinsic membrane absorption parameters in the intestine in situ. Pharm. Res. 6:S-91 (1989).
D. M. Oh, P. J. Sinko, and G. L. Amidon. Peptide transport of β-lactam antibiotics: structural requirements for an α-amino group. Pharm. Res. 7:S-119 (1990).
A. Tsuji, H. Hirooka, I. Tamai, and T. Terasaki. Evidence for a carrier-mediated transport system in the small intestine available for FK089, a new cephalosporin antibiotic without an amino group. J. Antibio. 39:1592–1597 (1986).
I. Tamai, H. Y. Ling, S. M. Timbul, J. Nishikido, and A. Tsuji. Stereospecific absorption and degradation of cephalexin. J. Pharm. Pharmacol. 40:320–324 (1988).
S. Yee and G. L. Amidon. Intestinal absorption mechanism of three angiotensin-converting enzyme inhibitors: Quinapril, benazeprdil and CGS16617. Pharm. Sci. 7:S-155 (1990).
S. Yokohama, T. Yoshioka, K. Yamashita, and N. J. Kitamori. Intestinal absorption mechanisms of thyrotropin-releasing hormone. Pharm. Dyn. 7:445–451 (1984).
M. J. Humphrey and P. S. Ringrose. Peptides and related drugs: A review of their absorption, metabolism, and excretion. Drug Metab. Rev. 17:283–310 (1986).
W. Kramer, F. Girbig, U. Gutjahr, H. W. Kleemann, I. Leipe, H. Urbach, and A. Wagner. Interaction of renin inhibitors with the intestinal uptake system for oligopeptides and β-lactam antibiotics. Biochim. Biophys. Acta 1027:25–30 (1990).
B. G. Munck. Intestinal absorption of amino acids. In L. R. Johnson (ed.), Physiology of the Gastrointestinal Tract, Raven Press, New York, 1981, p. 1097.
G. Esposito. In T. Z. Csaky (ed.), Pharmacology of Intestinal Permeation I, Springer-Verlag, Berlin, Heidelberg, 1984, p. 567.
D. N. Wade, P. T. Mearrick, and J. I. Morris. Active transport of L-dopa in the intestine. Nature 242:463–465 (1972).
M. Hu, P. Subramanian, H. I. Mosberg, and G. L. Amidon. Use of the peptide carrier system to improve intestinal absorption of L-α-methyldopa: Carrier kinetics, intestinal permeabilities, and in vitro hydrolysis of dipeptidyl derivatives of L-α-methyldopa. Pharm. Res. 6:66–70 (1989).
M. Merino, I. L. Peris-Ribera, F. Torres-Molina, A. Sánchez-Picó, M. C. García-Carbonell, V. G. Casabó, A. Martín-Villodre, and J. M. Plaá-Delfina. Evidence for a specialized transport mechanism for the intestinal absorption of baclofen. Biopharm. Drug Dispos. 10:279–297 (1989).
A. E. Merfeld, A. R. Mlodozeniec, M. A. Cortese, J. B. Rhodes, J. B. Dressman, and G. L. Amidon. The effect of pH and concentration on α-methyldopa absorption in man. J. Pharm. Pharmacol. 38:815–822 (1986).
N. Border, K. B. Sloan, and T. Higuchi. Improved delivery through biological membrane. 4. Prodrugs of L-dopa. J. Med. Chem. 20:1435–1445 (1977).
D. Burston, J. M. Addison, and D. M. Matthews. Uptake of dipeptides containing basic and acidic amino acids by rat small intestine in vitro. Clin. Sci. 43:823–837 (1972).
P. F. Bai, M. Hu, P. Subramanian, H. I. Mosberg, and G. L. Amidon. Utilization of peptide carrier system to improve the intestinal absorption: Targeting prolidase as a prodrug converting enzyme. J. Pharm. Sci. 80:1–4 (1991b).
Y. S. Kim. Intestinal mucosal hydrolysis of proteins and peptides. In K. Elliot and M. O'Connor (eds.), Peptide Transport and Hydrolysis, Ciba Foundation Symposium, Associated Scientific, Amsterdam, 1977, p. 151.
E. E. Sterchi and J. F. Woodley. Peptide hydrolases of the human small intestinal mucosa: Distribution of activities between brush border membranes and cytosol. Clin. Chim. Acta 102:49–56 (1980).
S. Miura, I. S. Song, A. Morita, R. H. Erickson, and Y. S. Kim. Distribution and biosynthesis of aminopeptidase N and dipeptidyl aminopeptidase IV in rat small intestine. Biochim. Biophys. Acta 761:66–75 (1983).
T. Q. Garvey, P. E. Hyman, and K. J. Isselbacher. γ-Glutamyl transpeptidase of rat intestine: Localization and possible role in amino acid transport. Gastroenterology 71:778–785 (1976).
M. Yoshioka, R. H. Erickson, J. F. Woodley, R. Gulli, D. Guan, and Y. S. Kim. Role of rat intestinal brush-border membrane angiotensin-converting enzyme in dietary protein digestion. Am. J. Physiol. 253:G781–G786 (1987).
H. Skovbjerg. Immunoelectrophoretic studies on human small intestinal brush border proteins—the longitudinal distribution of peptidases and disaccharidases. Clin. Chim. Acta 112:205–212 (1981).
N. Triadou, J. Bataille, and J. Schmitz. Longitudinal study of the human intestinal brush border membrane proteins: Distribution of the main disacharidases and peptidases. Gastroenterology 85:1326–1332 (1983).
S. Auricchio, L. Greco, B. D. G. Vizia, and V. Buonocore. Dipeptidylaminopeptidase and carboxypeptidase activities of the brush border of rabbit small intestine. Gastroenterology 75:1073–1079 (1978).
R. K. Kania, N. A. Santiago, and G. M. Gray. Intestinal surface amino-oligopeptidases. II. Substrate kinetics and topography of the active site. J. Biol. Chem. 252:4929–4934 (1977).
A. J. Kenny and S. Maroux. Topology of microvillar membrane hydrolases of kidney and intestine. Physiol. Rev. 62:91–128 (1982).
Y. S. Kim and E. J. Brophy. Rat intestinal brush border membrane peptidases. I. Solubilization, purification, and physicochemical properties of two different forms of the enzyme. J. Biol. Chem. 251:3199–3205 (1976).
Y. S. Kim, E. J. Brophy, and J. A. Nicholson. Rat intestinal brush border membrane peptidases. II. Enzymatic properties, immunochemistry and interactions with lectins of two different forms of the enzyme. J. Biol. Chem. 251:3206–3212 (1976).
S. T. Suresh. Microvillus membrane peptidases that catalyze hydrolysis of cysteinylglycine and its derivatives. In Methods in Enzymology, Academic Press, New York, 1985, Vol. 113, p. 471.
D. I. Friedman and G. L. Amidon. Oral absorption of peptides: Influence of pH and inhibitors on the intestinal hydrolysis of leu-enkephalin and analogues. Pharm. Res. 8:93–96 (1991).
N. Tobey, W. Heizer, R. Yeh, T. I. Huang, and C. Hoffner. Human intestinal brush border peptidases. Gastroenterology 88:913–926 (1985).
R. Walter, W. H. Simmons, and T. Yoshimoto. Proline specific endo-and exopeptidases. Mol. Cell. Biochem. 30:111–127 (1980).
B. Svensson, M. Danielsen, M. Staun, L. Jeppesen, O. Noren, and H. Sjostrom. An amphiphilic form of dipeptidyl peptidase IV from pig small-intestinal brush-border membrane. Eur. J. Biochem. 90:489–498 (1978).
J. Lasch, R. Koelsch, A. M. Ladhoff, and B. Hartrodt. Is the proline-specific aminopeptidase P of the intestinal brush border an integral membrane enzyme? Biomed. Biochim. Acta 45:833–843 (1986).
E. J. Holtzman, G. Pillay, T. Rosenthal, and A. Yaron. Aminopeptidase P activity in rat organs and human serum. Anal. Biochem. 162:476–484 (1987).
J. Lasch, R. Koelsch, T. Steinmetzer, U. Neumann, and H. U. Demuth. Enzymic properties of intestinal aminopeptidase P: A new continuous assay. FEBS 227:171–174 (1988).
W. Sidorowicz, J. Szechinski, P. C. Canizaro, and F. J. Behal. Cleavage of the Arg1-Pro2 bond of bradykinin by a human lung peptidase: Isolation, characterization, and inhibition by several β-lactam antibiotics (41828). Proc. Soc. Exp. Biol. Med. 175:503–509 (1984).
M. Yoshioka, R. H. Erickson, and Y. S. Kim. Digestion and assimilation of proline-containing peptides by rat intestinal brush border membrane carboxypeptidases. J. Clin. Invest. 81:1090–1095 (1988).
B. R. Stevens, A. Fernandez, C. Kneer, J. J. Cerda, M. I. Phillips, and E. R. Woodward. Human intestinal brush border angiotensin-converting enzyme activity and its inhibition by antihypertensive ramipril. Gastroenterology 94:942–947 (1988).
H. Yokosawa, S. Endo, Y. Ohgaki, J. I. Maeyama, and S. I. Ishii. Hydrolysis of substance p and its analogs by angiotensin-converting enzyme from rat lung: Characterization of endopeptidase activity of the enzyme. J. Biochem. 98:1293–1299 (1985).
E. M. Danielsen, J. P. Vyas, and A. J. Kenny. A neutral endopeptidase in the microvillar membrane of pig intestine. Biochem. J. 191:645–648 (1980).
N. W. Bunnett, A. J. Turner, J. Hryszko, R. Kobayashi, and J. H. Walsh. Isolation of endopeptidase-24.11 (EC 3.4.24.11, “enkephalinase”) from the pig stomach. Gastroenterology 95:952–957 (1988).
R. A. Skidgel and E. G. Erdös. Novel activity of human angiotensin I converting enzyme: Release of the NH2-and COOH-terminal tripeptides from the luteininzing hormone-releasing hormone. Proc. Natl. Acad. Sci. 82:1025–1029 (1985).
C. Doumeng and S. Maroux. Aminopeptidase, a cytosol enzyme from rabbit intestinal mucosa. Biochem. J. 177:801–808 (1979).
F. Endo, I. Matsuda, A. Ogata, and S. Tanaka. Human erythrocyte prolidase and prolidase deficiency. Pediat. Res. 16:227–231 (1982).
G. Mano, P. Nassi, G. Cappugi, G. Camici, and G. Ramponi. Swine kidney prolidase: Assay, isolation procedure, and molecular properties. Physiol. Chem. Phys. 4:75–87 (1972).
H. Sjöström and O. Norén. Structural properties of pig intestinal proline dipeptidase. Biochim. Biophys. Acta 359:177–185 (1974).
T. Yoshimoto, F. Matsubara, E. Kawano, and D. Tsuru. Prolidase from bovine intestine: Purification and characterization. J. Biochem. 94:1889–1896 (1983).
L. N. Lin and J. F. Brandts. Evidence suggesting that some proteolytic enzymes may cleave only the trans form of the peptide bond. Biochemistry 18:43–47 (1979).
I. Myara, C. Charpentier, and A. Lemonnier. Minireview: Prolidase and prolidase deficiency. Life Sci. 34:1985–1998 (1984).
J. F. Lenney, P. W. H. Chan, R. P. George, C. M. Kucera, G. S. Rinzler, and A. M. Weiss. (1982). Human-serum carnosinase, and activation by cadmium. Clin. Chim. Acta 123:221–231 (1982).
J. F. Lenney, R. P. George, S. C. Peppers, and C. M. Kuceraorallo. Characterization of human-tissue carnosine. Biochem. J. 228:653–660 (1985).
S. C. Peppers and J. Lenney. Bestatin inhibition of human tissue carnosinase, a non-specific cytosolic dipeptidase. Biol. Chem. Hoppe-Seyler 368:1281–1286 (1988).
B. Colas and S. Maroux. Simultaneous isolation of brush border and basolateral membrane from rabbit enterocytes: Presence of brush border hydrolases in the basolateral membrane of rabbit enterocytes. Biochim. Biophys. Acta 600:406–420 (1980).
R. Nau, G. Schäfer, and J. M. Colon. Proteolytic inactivation of substance P in the epithelial layer of the intestine. Biochem. Pharmacol. 34:4019–4023 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bai, J.P.F., Amidon, G.L. Structural Specificity of Mucosal-Cell Transport and Metabolism of Peptide Drugs: Implication for Oral Peptide Drug Delivery. Pharm Res 9, 969–978 (1992). https://doi.org/10.1023/A:1015885823793
Issue Date:
DOI: https://doi.org/10.1023/A:1015885823793