Abstract
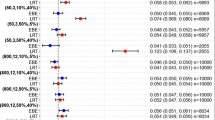
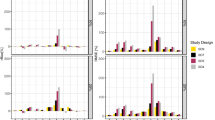
The objectives of this study were to assess the difference between actual and nominal significance levels, as judged by the likelihood ratio test, for hypothesis tests regarding covariate effects using NONMEM, and to study what factors influence these levels. Also, a strategy for obtaining closer agreement between nominal and actual significance levels was investigated. Pharmacokinetic (PK) data without covariate relationships were simulated from a one compartment iv bolus model for 50 individuals. Models with and without covariate relationships were then fitted to the data, and differences in the objective function values were calculated. Alterations were made to the simulation settings; the structural and error models, the number of individuals, the number of samples per individual and the covariate distribution. Different estimation methods in NONMEM were also tried. In addition, a strategy for estimating the actual significance levels for a specific data set, model and parameter was investigated using covariate randomization and a real data set. Under most conditions when the first-order (FO) method was used, the actual significance level for including a covariate relationship in a model was higher than the nominal significance level. Among factors with high impact were frequency of sampling and residual error magnitude. The use of the first-order conditional estimation method with interaction (FOCE-INTER) resulted in close agreement between actual and nominal significance levels. The results from the covariate randomization procedure of the real data set were in agreement with the results from the simulation study. With the FO method the actual significance levels were higher than the nominal, independent of the covariate type, but depending on the parameter influenced. When using FOCE-INTER the actual and nominal levels were similar. The most important factors influencing the actual significance levels for the FO method are the approximation of the influence of the random effects in a nonlinear model, a heteroscedastic error structure in which an existing interaction between interindividual and residual variability is not accounted for in the model, and a lognormal distribution of the residual error which is approximated by a symmetric distribution. Estimation with FOCE–INTER and the covariate randomization procedure provide means to achieve agreement between nominal and actual significance levels.
Similar content being viewed by others
REFERENCES
S. Vozeh, J. L. Steimer, M. Rowland, P. Morselli, F. Mentré, L. P. Balant, and L. Aarons. The use of population pharmacokinetics in drug development. Clin. Pharmacokinet. 30:81–93 (1996).
S. L. Beal and L. B. Sheiner (eds.). NONMEM Users Guides, University of California, San Francisco, CA, 1992.
L. B. Sheiner and S. L. Beal. A note on confidence intervals with extended least squares parameter estimates. J. Pharmacokin. Biopharm. 15:93–98 (1987).
D. B. White, C. A. Walawander, Y. Tung, and T. H. Grasela. An evaluation of point and interval estimates in population pharmacokinetics using NONMEM analysis. J. Pharmaco-kin. Biopharm. 19:87–112 (1991).
D. B. White, C. A. Walawander, D. Y. Liu, and T. H. Grasela. Evaluation of hypothesis testing for comparing two populations using NONMEM analysis. J. Pharmacokin. Biopharm. 20:295–313 (1992).
S. L. Beal and L. B. Sheiner. Estimating population kinetics. Crit. Rev. Biomed. Eng. 8:195–222 (1982).
M. Davidian and D. M. Giltinan. Nonlinear Models for Repeated Measurement Data. Monographs on Statistics and Applied Probability 62, Chapman and Hall, London, 1995.
S. L. Beal. Population pharmacokinetic data and parameter estimation based on their first two statistical moments. Drug Metab. Rev. 15:173–193 (1984).
S. L. Beal and L. B. Sheiner (eds.). NONMEM Users Guide—Part VII, University of California, San Fransisco, CA, 1998.
B. F. J. Manly. Randomization, Bootstrap and Monte Carlo Methods in Biology. Texts in Statistical Science, Chapman and Hall, London, 1997.
M. O. Karlsson and L. B. Sheiner. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J. Pharmacokin. Biopharm. 21:735–750 (1993).
E. N. Jonsson and M. O. Karlsson. Automated covariate model building within NON-MEM. Pharm. Res. 15:1463–1468 (1998).
J. W. Mandema, D. Verotta, and L. B. Sheiner. Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J. Pharmacokin. Biopharm. 20:511–528 (1992).
M. O. Karlsson, S. L. Beal, and L. B. Sheiner. Three new residual error models for popu-lation PK/PD analyses. J. Pharmacokin. Biopharm. 23:651–672 (1995).
Rights and permissions
About this article
Cite this article
Wählby, U., Jonsson, E.N. & Karlsson, M.O. Assessment of Actual Significance Levels for Covariate Effects in NONMEM. J Pharmacokinet Pharmacodyn 28, 231–252 (2001). https://doi.org/10.1023/A:1011527125570
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1011527125570