Abstract
Purpose
Local anesthetics in their therapeutic concentration range cause a vacuolar cytopathology that has been observed in vivo and in various types of mammalian cells. We examined whether active concentration ranges of drugs and the kinetics of the vacuolar response are clinically relevant and whether this phenomenon is associated with cytotoxicity, autophagy, and cell stress signalling.
Methods
We compared procaine and lidocaine for morphological, functional, and signalling responses in a previously exploited non-neuronal system, primary smooth muscle cells. Several markers conjugated to fluorescent proteins allowed morphological and functional analysis of vacuolar cells. Signalling related to autophagy and cell stress was addressed (immunoblotting of cell lysates).
Results
Within 2–4 hr, lidocaine and procaine (≥1 mM) induced massive cell vacuolization, a response abated by the V-ATPase inhibitor, bafilomycin A1, and activated macroautophagic signalling (LC3 II formation) but not other stress signalling (p38, ERK1/2, p53, no influence on serum-controlled Akt phosphorylation). Novel aspects of the morphological analysis include reduced LC3 labelling of the large vacuoles in cells treated with 3-methyl-adenine, inhibition of CD63 labelling of these vacuoles by co-expression of dominant negative Rab7, retention of secretory green fluorescent protein (GFP) possessing a signal sequence in vacuolar cells, and partial vacuole labelling with lysosomal-associated membrane protein 1 (LAMP1). Lidocaine (2.5–5 mM) was not overtly cytotoxic but arrested cell division over 48 hr.
Conclusions
V-ATPase-mediated sequestration of clinically relevant concentrations of local anesthetics sequentially involves vacuolization, macroautophagic signalling, and lysosome fusion to large vacuoles. Disruption of the secretory pathway and mitotic arrest were also observed over several hours without major cytotoxicity.
Résumé
Objectif
À concentration thérapeutique, les anesthésiques locaux causent une cytopathologie vacuolaire qui a été observée in vivo et dans divers types de cellules en culture. Nous avons examiné si les concentrations actives de médicaments et la cinétique de cette réponse sont cliniquement pertinentes et si ce phénomène est associé à la cytotoxicité, l’autophagie et la signalisation de stress cellulaire.
Méthode
On a comparé les réponses morphologiques, fonctionnelles et signalétiques à la procaïne et la lidocaïne dans un système non neuronal préalablement exploité, le muscle lisse en culture primaire, afin d’éclairer leurs effets toxicologiques à des niveaux thérapeutiques. Plusieurs marqueurs conjugués à des protéines fluorescentes ont permis des analyses morphologiques et fonctionnelles des cellules vacuolaires. La signalisation autophagique et celle reliée au stress cellulaire ont été évaluées (immunobuvardage de lysats cellulaires).
Résultats
La lidocaïne et la procaïne (≥1 mM) induisent en 2-4 hr une vacuolisation cellulaire massive, une réponse fortement réduite par l’inhibiteur de l’ATPase vacuolaire, la bafilomycine A1, et activent la signalisation macro-autophagique (formation de LC3 II) mais pas d’autres voies de stress (p38, ERK1/2, p53; pas d’influence sur la phosphorylation d’Akt contrôlée par le sérum). Les nouveaux aspects de l’analyse morphologique incluent la réduction du marquage LC3 des vacuoles géantes par un traitement à la 3-méthyl-adénine, l’inhibition du marquage CD63 de ces vacuoles par la co-expression de Rab7 dominant négatif, la rétention de la GFP sécrétoire dans les cellules vacuolaires et un marquage partiel des vacuoles par LAMP1. La lidocaïne (2.5-5 mM) n’est pas cytotoxique mais arrête la division cellulaire en 48 hr.
Conclusion
La séquestration de concentrations cliniquement pertinentes d’anesthésiques locaux est médiée par la V-ATPase et comporte séquentiellement la vacuolisation, la signalisation macro-autophagique et la fusion lysosomale aux vacuoles géantes; d’autres réponses concernent une perturbation de la voie sécrétoire et un arrêt mitotique sans cytotoxicité majeure sur une période de plusieurs heures.
Similar content being viewed by others
Introduction
Local anesthetics are the drugs that reversibly block nerve conduction when injected into an anatomically confined region. They generally possess two chemical domains: a hydrophilic one, usually a tertiary or secondary amine, and a hydrophobic moiety based on a benzene ring.1 They influence nerve conduction by accessing an intracellular site in voltage-operated sodium channels1; thus, simple diffusion of the uncharged form of the drug through the plasma membrane is a postulated step for their action (Fig. 1). In nonneuronal cells bordering the site of the administration of local anesthetics in humans and animals, an “off-target” vacuolar cytopathology has been described. Lidocaine 1% (42 mM) in the anterior chamber of the eye caused endothelial, fibroblast, and microvascular cell vacuolization, inflammation, and extensive drug uptake.2 – 4 Intracameral administration of the alternate agent, ropivacaine (0.1–1%), also induced vacuolization of corneal endothelial cells and mitochondrial damage (electron microscopy).5 The topical formulation, EMLA (2.5% lidocaine, 2.5% prilocaine), occasionally causes a histopathologic reaction that includes focal vacuolization in the epidermal and other nonneuronal cell types (histiocytes, endothelial cells, fibroblasts, pericytes).6 – 8 In vitro, procaine and lidocaine cause massive vacuolization at millimolar concentrations in various cell types.9 – 11
Schematic representation of the anticipated cellular reactions induced by local anesthetics (A) that are off target relative to voltage dependent Na+ channels. See Introduction for details
Vacuolar (V)-ATPase is the enzyme complex necessary for the acidification of some intracellular compartments (trans-Golgi network, endosomes, lysosomes, secretory granules), and protonated amines may be trapped into these vacuoles at low pH. The reversible vacuolar state of cells induced by local anesthetics may conform to the following model inspired from the recent study of other weak bases (Fig. 1).11 – 15 The V-ATPase-dependent trapping of the cationic form of the drugs in acidic vesicles is sequentially followed by their osmotic swelling (Step 1), their macroautophagic envelopment (Step 2), and their maturation with lysosome fusion (Step 3) with progressive acquisition of marker proteins typical of autophagy (e.g., LC3) and late endosomes–lysosomes (e.g., CD63) by vacuoles. Previous morphological investigations mostly based on procainamide effects showed that the subcellular origin of a fraction of the giant vacuoles was the trans-Golgi.15 , 16 Mitotic arrest without overt toxicity was also observed in vacuolar cells.15 – 18
The present experiments aim to characterize the cell morphological and toxic responses to local anesthetics relative to the vacuolar-autophagic state. Novel questions addressed include the clinical relevance of drug concentrations that cause this response and its time frame, the relationship of the vacuolar response to cytotoxicity, the experimental dissociation of the three postulated steps of the vacuolar response (Fig. 1), and exploration of stress signalling in vacuolar cells. Two drugs were selected in relationship with the chemical classification of local anesthetics, i.e., the widely used amide, lidocaine, and the ester, procaine. A previously exploited primary cell model of nonneuronal origin, rabbit vascular smooth muscle cells,15 , 17 , 19 was used to address the cellular effects of local anesthetics. These cells of mesenchymatous origin are related to pericytes known to be morphologically affected in vivo by local anesthetics,11 are well adapted to morphological analysis (well spread, very adherent, transfectable), and may be used to represent the wide variety of nonneuronal cells affected in tissues by these drugs.
Materials and methods
Drugs
Bafilomycin A1 was purchased from either LC Laboratories (Woburn, MA, USA) or Sigma–Aldrich (St. Louis, MO, USA). All other drugs were obtained from Sigma–Aldrich. Bafilomycin A1 was dissolved in dimethyl sulfoxide (DMSO) (Final concentration is always inferior to 0.1% v/v), and control cells were treated with the DMSO vehicle.
Cells
A local ethics committee approved procedures based on rabbits, and the animals were cared for in accordance with the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care). Primary cultures of smooth muscle cells were obtained from explants of de-endothelialized rabbit aorta maintained in medium 199 supplemented with antibiotics, L-glutamine, and 10% fetal bovine serum on gelatin-coated surfaces and propagated for up to five passages.
Microscopic techniques
The vacuolization of cultured cells, photographed in phase contrast in a manner to observe clear vacuoles on a dark background, was quantified as the percent of individual cell pixels above a set threshold after increasing the contrast of the picture in a standardized manner for each cell line (Photoshop software).13 These numerical values were averaged and compared using non-parametric statistical tests.
Expression vectors were transiently transfected overnight (20–22 hr) using the ExGen reagent (Fermentas) used as directed. Expression vectors for GFP-Rab7, GFP-Rab5, and GFP-CD63 were previously described.15 , 17 Cherry fluorescent protein (CherryFP)-conjugated Rab7 or its dominant negative guanosine diphosphate-locked (GDP-locked) mutant N125I were prepared by recloning the insert from the GFP-conjugated construction into the appropriate vector. GFP-LC3 labels autophagosomes in mammalian cells18; the pEGFP-LC3 expression vector for this chimerical protein was a generous gift from Dr. T. Yoshimori (Osaka University, Japan). GFP-golgin-97, coded by the vector GRIP1220 associates with the cytosolic face of the trans-Golgi network (gift from Dr. Sean Munro, Medical Research Council Laboratory of Molecular Biology, Cambridge, UK). Secretory enhanced GFP, encoded in the s-EGFP vector (generous gift from Dr. Paul Joyce, Concordia University, Montreal, QC, Canada) contains a N-terminal signal sequence that targets it to the secretory pathway.21 LAMP1-mEGFP is a vector coding for a GFP-conjugated lysosomal marker22 (generous gift from Dr. Esteban C. Dell’Angelica, UCLA Department of Human Genetics).
Proliferation assay
The effect of lidocaine on smooth muscle cell proliferation over 72 hr (drug present for the last 48 hr) was determined precisely as described.11 At the end, viability was tested using trypan blue exclusion (Invitrogen) used as instructed dissolved in Hank’s buffered salt solution.
Immunoblots
Anti-human LC3B rabbit polyclonal antibodies (Novus; dilution 1:3,000) were used to reveal the cytosolic form LC3-I and processed form LC3-II in total smooth muscle cell extracts (5–15 μg · track−1) run on 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (β-actin immunoblot to document equal loading).15 The signalling studies were extended to the immunoblotting of the phosphorylated (activated) forms of the mitogen-activated protein (MAP) kinases p38 and ERK1/2 and Akt (total ERK1/2, total Akt and β-actin used for showing equal loading of tracks). Samples were separated on 9% SDS–PAGE. The sources of the antibodies and their dilution were: monoclonal anti-phospho-p38 (Cell Signaling Technologies, Inc., Beverly, MA, USA) 1:1,000; monoclonal anti-phospho-ERK1/2, Cell Signaling Technologies, 1:1,000; polyclonal anti-total ERK1/2, Cell Signaling Technologies; polyclonal anti-phospho-Akt (Ser 473), Cell Signaling Technologies, 1:1,000; polyclonal anti-AKT, Cell Signaling Technologies, 1:1,000; monoclonal anti-β-actin, Sigma–Aldrich, 1:50,000.
Statistical analysis
Numerical results are expressed as mean ± standard error of mean (SEM). Calculations were performed using the GraphPad Instat 3.0 program. Nonnormally distributed groups of values were analyzed using nonparametric analysis of variance (ANOVA) (Kruskall–Wallis test) followed by Dunn’s multiple comparison test. Normal sets of values were compared using ANOVA followed by Tukey–Kramer test for multiple comparisons. The effect of drugs in the trypan blue viability test was evaluated using χ2-test.
Results
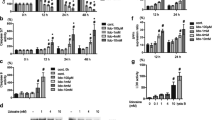
Treatment with buffered solutions (pH 7.4) of lidocaine or procaine (1–5 mM) determined a statistically significant and concentration-dependent vacuolization of the smooth muscle cells (Fig. 2a, sample photographic records after 4 hr of treatment; Fig. 2b, proportion of the average cell surface occupied by clear vacuoles as a function of drug concentration). The vacuoles were barely detectable before 1 hr of treatment (data not shown). The vacuolization induced by lidocaine or procaine (2.5 mM, 2 hr), less intense than after 4-hr treatment and protracted for lidocaine, was virtually abolished by co-treatment with the specific V-ATPase inhibitor, bafilomycin A1 (Fig. 3). This also applies to lidocaine- or procaine-induced vacuolization over 4–24 hr19 (and data not shown).
Massive vacuolization of rabbit aortic smooth muscle cells induced by some local anesthetics. a Phase contrast observation of cells treated for 4 hr with buffered (pH 7.4) drugs introduced into the regular culture medium (original magnification 100×). b Quantification of the vacuolization as a percent of individual cell surface pixels above a set threshold following image treatment (see Materials and methods). The 0 mM concentration value conventionally represents the percent of pixels above a set threshold in saline-treated control cells. The number of evaluated cells is indicated above each bar. Kruskall–Wallis test showed that the values were significantly heterogeneous (P < 0.001). Comparison of each data set with control (zero concentration) by Dunn’s multiple comparison test: * P < 0.001
Vacuolization of smooth muscle cells by lidocaine and procaine (2 hr) is V-ATPase-dependent. a Phase contrast microscopy, original magnification 100×. b Vacuolization quantification. Values are means ± SEM. The number of cells evaluated is indicated above each bar. Kruskall–Wallis test showed that the values were significantly heterogeneous (P < 0.001). Dunn’s multiple comparison test indicated that all pairs of value differed significantly from each other (P < 0.001) except those indicated by the symbol “*”. SEM standard error of mean
Vacuoles induced by procaine or lidocaine (4 hr) were decorated mostly at their periphery with GFP-conjugated markers of late endosomes–lysosomes (Rab7, CD63) and with the autophagy effector (GFP-LC3) in smooth muscle cells (Fig. 4). The labelling of cytoplasmic particles by the latter fusion protein was rare in control cells; whereas small cytosolic particles positive for either GFP-Rab7 or GFP-CD63 were seen. Secretory GFP (s-EGFP) granular labelling was weak in control smooth muscle cells (Fig. 4), but the conditioned culture medium was fluorescent, consistent with the secretion of this protein. Cells made vacuolar with procaine or lidocaine retained more fluorescence, usually outside the large vacuoles seen in negative, but some peripheral vacuoles were occasionally filled with a highly fluorescent solution (more frequent with procaine, epifluorescence microscopy). LAMP1-mEGFP, coding for a lysosomal protein, was distributed in many but not all large vacuoles induced by treatment with either local anesthetic (Fig. 4).
LC3 is processed from a cytosolic type I, ~18 kDa form to a ~16-kDa, autophagosome-bound and lipidated type II. Control smooth muscle cells expressed very little endogenous LC3 I and no detectable LC3 II (Fig. 5a). Both local anesthetics caused the conversion of LC3 at and above 1 mM, and the LC3 II signal was considerably more intense than the LC3 I. Co-treatment with 3-methyl-adenine (10 mM), an inhibitor of class III phosphatidylinositol-3-kinase necessary for autophagic envelopment,23 significantly abated the accumulation of LC3 II induced by lidocaine in the cell lysate (2.5 mM) (Fig. 5b).
Processing of LC3 in smooth muscle cells subjected to the indicated treatments for 4 hr. β-actin immunoblot was also performed to document equal loading. a Effect of procaine or lidocaine concentration. Representative results of two experiments. b Processing of LC3 in smooth muscle cells subjected to lidocaine treatment as modified by the autophagic inhibitor 3-methyl-adenine. Immunoblot representative of three experiments, above which, the histograms show the mean densitometric values ± SEM of the three determinations. Values for LC3 I were not different, but ANOVA indicated that LC3 II values were heterogeneous (P < 0.001). * P < 0.001 vs control; † P < 0.001 vs lidocaine alone, Tukey–Kramer multiple comparison test. SEM standard error of mean
Morphological studies were extended to cells co-transfected with vectors coding for GFP- and cherryFP-conjugated proteins (Fig. 6). The occurrence of endosomal labelling in lidocaine-induced vacuoles was evaluated in cells expressing cherryFP-Rab7 and GFP-Rab5 (Fig. 6). Few of the large vacuoles were labelled with Rab5, but most were positive for Rab7. There was practically no colocalization of both Rab constructions in either control or lidocaine-treated cells. By contrast, double labelling of lidocaine-induced giant vacuoles with GFP-LC3 and cherryFP-Rab7 was frequent (Fig. 6). Pretreating the cells with 3-methyl-adenine inhibited efficiently GFP-LC3 labelling of large vesicles, but only infrequently that of Rab7-cherryFP (Fig. 6, arrowhead).
Morphology of co-expressed GFP- and Cherry FP-conjugated proteins, as indicated at the left of each row, in representative control or lidocaine-treated (2.5 mM, last 4 hr) smooth muscle cells. Some cells were co-treated with 3-methyl-adenine (10 mM, applied 5 hr before observation), as indicated. Original magnification 1,000×. GFP green fluorescent protein
The two late endosome-lysosome tags, GFP-CD63 and cherryFP-Rab7, co-localized in several lidocaine-induced giant vacuoles (Fig. 6, arrowheads). However, the expression of a dominant negative cherryFP-Rab7 construction inhibited labelling of the lidocaine-induced vacuoles by coexpressed GFP-CD63, supporting the dependence of CD63 labelling on lysosomal fusion to giant vacuoles. Smooth muscle cells co-transfected with vectors coding for cherryFP-Rab7 and GFP-golgin-97 were treated for 4 hr with lidocaine 2.5 mM (Fig. 6). While there was no overt colocalization of both proteins, a fraction of the Rab7-positive giant vacuoles contained Golgi remnants, some swollen, labelled with the green fusion protein.
It was verified that lidocaine, like other amines that cause cell vacuolar response, induced a mitotic arrest in a smooth muscle cell proliferation assay at relevant concentrations over 48 hr (Fig. 7). At and above 2.5 mM, the drug significantly inhibited the growth above the number of seeded cells (50,000). The inhibitory effect of 1 mM lidocaine was not significant, but the vacuolar response to this concentration of amine was not sustained for 48 hr in most cells (possibly related to slow drug metabolism). By contrast, cells treated with 2.5 or 5 mM for 48 hr remained heavily vacuolar until counted, but mostly alive based on trypan blue exclusion (0.9% positive for trypan blue at 2.5 mM vs 0% for controls, not significant per χ2 test; 9.4% positive for 5 mM lidocaine; P < 0.001; 169–563 cells per condition). In this set of experiments, inhibition of organelle acidification with bafilomycin A1 (120 nM) exerted a similar antimitotic effect (46,166 ± 1,389 cells per dish after 48 hr).
Effect of lidocaine on the proliferation of rabbit smooth muscle cells. Fifty thousand cells were seeded in petri dishes 3 days before the counts, and the drugs were introduced for the last 48 hr. Values are means ± SEM of 5–6 determinations. The Kruskall–Wallis test indicated that the values were heterogeneous (P < 0.001). Dunn’s multiple comparison test vs control: * P < 0.05. SEM standard error of mean
After observing such profound morphological and functional effects of lidocaine on cultured cells, we probed additional stress signalling pathways (Fig. 8). In serum-starved smooth muscle cells (a condition that does not impair vacuolization), lidocaine or procaine (0.25–2.5 mM) failed to activate (phosphorylate) the MAP kinases, ERK1/2 or p38. A longer (24 hr) treatment with procaine was also negative. Positive controls were cells treated with epidermal growth factor (EGF) or anisomycin, respectively.24 In the present system, Akt phosphorylation responded to the presence of mitogens, being weak in serum-starved cells, restored by the short EGF treatment and strong in the presence of serum. Importantly, local anesthetics at concentration levels that do not (0.25 mM) or do elicit the vacuolar cytopathology (2.5 mM) failed to either induce or reduce the phospho-Akt signals in the absence or presence of serum, respectively (Fig. 8).
A cytotoxicity assay based on propidium iodide and fluorescein acetate staining of cells12 showed that both lidocaine and procaine were well tolerated over 24 hr up to 5 mM (data not shown). The drugs did not acutely deenergize mitochondria at 2.5 mM, as evaluated by a rhodamine 123 retention assay13 (data not shown).
Discussion
At millimolar concentrations, procaine and lidocaine recapitulate the vacuolar and macroautophagic cytopathology caused by other drugs like procainamide and H1 receptor agonists and antagonists,15 , 16 but with low cytotoxicity at and below 5 mM. Novel aspects of this work include the dissociation of vacuolization from autophagy with 3-methyl-adenine, lysosomal fusion inhibition with dominant negative Rab7, and extended investigation of cell stress signalling. In fact, the formation of giant vacuoles is a species- and cell type-independent response to concentrated amines that transcends conventional drug classes in cultured cells11 – 14 but sometimes occurs at supratherapeutic drug concentrations. Four novel questions were examined in the present study:
Clinical relevance of the drug concentrations and kinetics of the vacuolar response to local anesthetics
The threshold for a robust vacuolar and macroautophagic response was 1 mM for both lidocaine and procaine, which is a small fraction of the concentration of injected solutions. The 1 mM level can be maintained for at least 2 hr in the cerebrospinal fluid of patients subjected to lidocaine spinal anesthesia with periodic readministration.25 In another therapeutic application, a 5% (210 mM) lidocaine patch delivers the drug to large surface areas of the skin for 12–24 hr per day.26 In the so-called Mohs micrographic surgery for skin carcinomas, lidocaine is heavily used and readministered over several hours.27 Drug measurement in the extracellular fluid may be misleading to a certain extent, because local anesthetics are rapidly taken up by cells. The presence of massive vacuolization in various cell types in the skin treated with topical lidocaine-prilocaine is a testimony for the clinical relevance of the response (see Introduction). The actual presence of the amines in the enlarged vacuoles has been shown for pigmented or fluorescent cationic drugs in previous reports11 , 15 , 16 , 28 and is a reasonable interpretation for lidocaine and procaine (Fig. 2), molecules that do not support fluorescence imaging.
Relationship of the vacuolar response to cytotoxicity
A concentration level of either lidocaine or procaine that induced a very intense vacuolization, 5 mM, was only associated with minimal and time-dependent cell death in 24–48 hr despite the spectacular morphological changes, suggesting that this reversible response is largely harmless. We have verified that lidocaine causes a mitotic arrest without any significant mortality at 2.5 mM in smooth muscle cells (48 hr treatment; Fig. 7), like other amines that induce vacuolization.11 , 14 The mechanism of this effect is currently unknown, but bafilomycin A1 alone elicits the same type of effect suggesting commonalities related to acid vacuoles. Antimitotic actions of local anesthetics may not be important for most clinical applications of these agents, rarely used for more than a few hours, although the 5% lidocaine patch is used continuously by some patients with neuropathies.26
Investigation of the origin and fate of giant vacuoles
We verified the V-ATPase-dependence of lidocaine- or procaine-induced cell vacuolization (Figs. 1 and 3). The GTPase Rab5 is only rarely detected in lidocaine-induced vacuoles in rabbit smooth muscle cells (Fig. 6) supporting that the vast majority of the large vacuoles are not (at least at the studied stage) of early endosomal nature. Evidence for a trans-Golgi origin of a fraction of the giant vacuoles induced by concentrated amines is supported by the presence of golgin-97-positive remnants in lidocaine-induced large vacuoles (Fig. 6) and a novel approach based on s-EGFP (Fig. 4). Indeed, either local anesthetic determined a cellular retention of this protein. In another cell type, a rat hepatoma, the retention of the normally secreted α-fetoprotein was a consequence of amine-induced vacuolization.12 The effects on the secretory pathway are largely overlooked in the literature where the amines are said to be lysosomotropic.29 A wide range of cellular functions that involve vacuolar trafficking, including endocytosis and secretion, are dependent on V-ATPase.30 The V-ATPase-driven vacuolization is followed by a transition to autophagosomes labelled with LC3 and with the late endosomes–lysosome markers, LAMP1, CD63, and Rab7, a logical association because macroautophagosomes fuse with lysosomes (Fig. 1).31 Inhibiting macroautophagic envelopment with 3-methyl-adenine logically reduced lidocaine-induced LC3 II formation (Fig. 5b) and GFP-LC3 labelling of giant vacuoles (Fig. 6). However, most vacuoles were still positive for Rab7 under these circumstances, suggesting that the origin of the majority of vacuoles was from a Rab7-positive stock (Fig. 1), such as the perinuclear late endosomes. This possibility is supported by time lapse photography of rabbit smooth muscle cells treated with procainamide. The vacuolization first appeared in a distinctively perinuclear area where late endosomes are found.11 A dominant negative (GDP-locked) Rab7 construction had the power to inhibit lysosome fusion to lidocaine-induced vacuoles, as estimated by CD63 presence in the vacuole membranes (Fig. 6). This approach to dissociate autophagy from lysosome fusion had previously been utilized.32
Exploration of cell stress signalling
Per se, amine-induced cell vacuolization is not associated with cell stress signalling in the present or previous investigations (no recruitment of NF-κB by procainamide or triethylamine,12 no activation of MAP kinases ERK1/2 or p38, Fig. 8). The p38 has originally been discovered as part of a signalling system responsive to osmotic stress,33 but the osmotic mechanisms that explain the protracted swelling of the vacuoles following amine uptake15 did not activate p38. Akt signalling is not stimulated nor inhibited during vacuolization induced by local anesthetics, failing to demonstrate a link between this phenomenon and starvation-induced autophagy controlled by the phosphatidylinositol-3-kinase/Akt/mTOR pathway of signalling.24 LC3 conversion into its lipidated form (II) occurs precisely at procaine or lidocaine concentrations that acutely induce vacuolization (Fig. 5a), suggesting that the osmotic swelling of these organelles is associated with membrane damage sufficient to initiate starvation-independent macroautophagy. Alternatively, at this threshold, the buffering of vacuolar acidity by trapped amine molecules and water may stop baseline autophagic digestion in cells with accumulation of LC3 and other markers.
Areas for further progress include the study of the effect of local anesthetic reservoir formation on their onset and duration of action in vivo, a problem that we have experimentally addressed for other pharmacologically active amines.13 Also, local anesthetics exert other forms of toxicity. Both amides, lidocaine and bupivacaine, are uncouplers of oxidative phosphorylation34 that collapse the pH gradient and electrical potential across the inner mitochondrial membrane.35 Consequently, lidocaine induces cell apoptosis or necrosis by recruiting the intrinsic mitochondrial pathway in neuronal or nonneuronal systems,35 – 37 but at concentrations higher than those used in this study (usually above 5 mM). However, this form of toxicity may be prominent for lower concentrations of the more lipophilic agent, bupivacaine.
In summary, local anesthetics induce the V-ATPase-mediated vacuolar cytopathology that accounts for previous observations in tissues (see Introduction) and is associated with generic effects, e.g., mitotic arrest, that are not likely to be significant during a short term clinical use, because the amine-induced vacuolization is fully reversible upon drug washout.11 , 14 , 15 The time course and concentration-effect relationship of the vacuolar cytopathology are relevant for specific clinical uses, and present results suggest a sequence of events where the V-ATPase-mediated concentration of drugs is followed by macroautophagy in swollen vacuoles and lysosomal fusion.
References
Catterall W, Mackie K. Local anesthetics. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. McGraw-Hill: New York; 2006. p. 369–86.
Kim T, Holley GP, Lee JH, Broocker G, Edelhauser HF. The effects of intraocular lidocaine on the corneal endothelium. Ophthalmology 1998; 105: 125–30.
Anderson NJ, Nath R, Anderson CJ, Edelhauser HF. Comparison of preservative-free bupivacaine vs. lidocaine for intracameral anesthesia: a randomized clinical trial and in vitro analysis. Am J Ophthalmol 1999; 127: 393–402.
Atilla H, Tekeli O, Can B, Karel F, Saran Y. Effects of intracameral lidocaine on ocular tissues. Clin Experiment Ophthalmol 2003; 31: 73–7.
Cakmak SS, Olmez G, Nergiz Y, Unlu K, Soker S. The effect of intracameral ropivacaine on the corneal endothelium. Jpn J Ophthalmol 2005; 49: 267–8.
Hoss DM, Gross EG, Grant-Kels JM. Histopathology of an adverse reaction to a eutectic mixture of the local anesthetics lidocaine and prilocaine. J Cutan Pathol 1999; 26: 100–4.
Vallance H, Chaba T, Clarke L, Taylor G. Pseudo-lysosomal storage disease caused by EMLA cream. J Inherit Metab Dis 2004; 27: 507–11.
Cazes A, Prost-Squarcioni C, Bodemer C, Heller M, Brousse N, Fraitag S. Histologic cutaneous modifications after the use of EMLA cream, a diagnostic pitfall: review of 13 cases. Arch Dermatol 2007; 143: 1074–6.
Miller K, Foster JR. Evidence of transient effect by lignocaine on alveolar macrophage morphology. J Immunol Methods 1981; 43: 163–8.
Michalik M, Pierzchalska M, Pabianczyk-Kulka A, Korohoda W. Procaine-induced enhancement of fluid-phase endocytosis and inhibition of exocytosis in human skin fibroblasts. Eur J Pharmacol 2003; 475: 1–10.
Morissette G, Moreau E, C–Gaudreault R, Marceau F. Massive cell vacuolization induced by organic amines such as procainamide. J Pharmacol Exp Ther 2004; 310: 395–406.
Morissette G, Moreau E, C-Gaudreault R, Marceau F. N-substituted 4-aminobenzamides (procainamide analogs): an assessment of multiple cellular effects concerning ion trapping. Mol Pharmacol 2005; 68: 1576–89.
Morissette G, Bouthillier J, Marceau F. Trapping of adrenergic decongestant drugs into cellular endomembrane compartments: toxicological and pharmacological consequences. Int Immunopharmacol 2007; 7: 1869–79.
Morissette G, Germain L, Marceau F. The antiwrinkle effect of topical concentrated 2-dimethylaminoethanol involves a vacuolar cytopathology. Br J Dermatol 2007; 156: 433–9.
Morissette G, Lodge R, Marceau F. Intense pseudotransport of a cationic drug mediated by vacuolar ATPase: procainamide-induced autophagic cell vacuolization. Toxicol Appl Pharmacol 2008; 228: 364–77.
Morissette G, Lodge R, Bouthillier J, Marceau F. Receptor-independent, vacuolar ATPase-mediated cellular uptake of histamine receptor-1 ligands: possible origin of pharmacological distortions and side effects. Toxicol Appl Pharmacol 2008; 229: 320–31.
Hunyady L, Baukal AJ, Gaborik Z, et al. Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J Cell Biol 2002; 157: 1211–22.
Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19: 5720–8.
Morissette G, Marceau F. Ô miroir, dis-moi comment les amines effacent les rides: la cytopathologie vacuolaire médiée par la V-ATPase. Med Sci (Paris) 2007; 23: 579–80.
Munro S, Nichols BJ. The GRIP domain—a novel Golgi-targeting domain found in several coiled-coil proteins. Curr Biol 1999; 9: 377–80.
Jain RK, Joyce PB, Molinete M, Halban PA, Gorr SU. Oligomerization of green fluorescent protein in the secretory pathway of the endocrine cells. Biochem J 2001; 360: 645–9.
Falcón-Pérez JM, Nazarian R, Sabatti C, Dell’Angelica EC. Distribution and dynamics of Lamp1-containing endocytic organelles in fibroblasts deficient in BLOC-3. J Cell Sci 2005; 118: 5243–55.
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007; 8: 741–52.
Gould GW, Cuenda A, Thomson FJ, Cohen P. The activation of distinct mitogen-activated protein kinase cascades is required for the stimulation of 2-deoxyglucose uptake by interleukin-1 and insulin-like growth factor-1 in KB cells. Biochem J 1995; 311: 735–8.
Mörch ET, Rosenberg MK, Truant AT. Lidocaine for spinal anesthesia. A study of the concentration in the spinal fluid. Acta Anaesthesiol Scand 2007; 51: 1005–15.
Gammaitoni AR, Alvarez NA, Galer BS. Safety and tolerability of the lidocaine patch 5%, a targeted peripheral analgesic: a review of the literature. J Clin Pharmacol 2003; 43: 111–7.
Robins P, Ashinoff R. Prolongation of anesthesia in Mohs micrographic surgery with 2% lidocaine jelly. J Dermatol Surg Oncol 1991; 17: 649–52.
Ouar Z, Bens M, Vignes C, et al. Inhibitors of vacuolar H+-ATPase impair the preferential accumulation of daunomycin in lysosomes and reverse the resistance to anthracyclines in drug-resistant renal epithelial cells. Biochem J 2003; 370: 185–93.
Zhang X, Zheng N, Rosania GR. Simulation-based cheminformatic analysis of organelle-targeted molecules: lysosomotropic monobasic amines. J Comput Aided Mol Des 2008; 22: 629–45.
Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 2008; 20: 415–26.
Jäger S, Bucci C, Tanida I, et al. Role of Rab7 in maturation of late autophagic vacuoles. J Cell Sci 2004; 117: 4837–48.
Gutierrez MG, Mufano DB, Beron W, Colombo MI. Rab7 is required for the normal progression of the autophagic pathway in mammalian cells. J Cell Sci 2004; 117: 2687–97.
Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 1994; 265: 808–11.
Wallace KB. Mitochondrial off targets of drug therapy. Trends Pharmacol Sci 2008; 29: 361–6.
Johnson ME, Uhl CB, Spittler KH, Wang H, Gores GJ. Mitochondrial injury and caspase activation by the local anesthetic lidocaine. Anesthesiology 2004; 101: 1184–94.
Werdehausen R, Braun S, Essmann F, et al. Lidocaine induces apoptosis via the mitochondrial pathway independently of death receptor signaling. Anesthesiology 2007; 107: 136–43.
Perez-Castro R, Patel S, Garavito-Aguilar ZV, et al. Cytotoxicity of local anesthetics in human neuronal cells. Anesth Analg 2009; 108: 997–1007.
Acknowledgements
We thank Ms. Johanne Bouthillier for technical help, Dr. Marc Pouliot (CHUQ-CHUL) for facilitating the access to microscopic equipment, and Ms. Lise-Andrée Gobeil and Drs. Robert Lodge and Michel J. Tremblay (Centre de recherche en Infectiologie, Centre Hospitalier Universitaire de Québec) for the gift of vectors containing cherryFP.
Funding sources
Supported by the Canadian Institutes of Health Research (operating grant MOP-74448 to F.M., Canada Graduate Scholarships Doctoral Award to G.M.) and Fonds de la recherche en Santé du Québec, QC, Canada (Studentship award to M.T.B.).
Competing interests
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is accompanied by an editorial. Please see Can J Anesth 2010; 57(3).
Rights and permissions
About this article
Cite this article
Bawolak, MT., Morissette, G. & Marceau, F. Vacuolar ATPase-mediated sequestration of local anesthetics in swollen macroautophagosomes. Can J Anesth/J Can Anesth 57, 230–239 (2010). https://doi.org/10.1007/s12630-009-9220-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-009-9220-9