Abstract
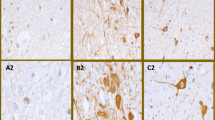
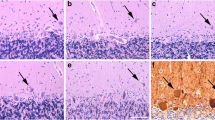
Clinicoanatomic correlation in the spinocerebellar ataxias (SCA) and Friedreich’s ataxia (FRDA) is difficult as these diseases differentially affect multiple sites in the central and peripheral nervous systems. A new way to study cerebellar ataxia is the systematic analysis of the “reciprocal cerebellar circuitry” that consists of tightly organized reciprocal connections between Purkinje cells, dentate nuclei (DN), and inferior olivary nuclei (ION). This circuitry is similar to but not identical with the “cerebellar module” in experimental animals. Neurohumoral transmitters operating in the circuitry are both inhibitory (γ-aminobutyric acid in corticonuclear and dentato-olivary fibers) and excitatory (glutamate in olivocerebellar or climbing fibers). Glutamatergic climbing fibers also issue collaterals to the DN. The present study applied five immunohistochemical markers in six types of SCA (1, 2, 3, 6, 7, 17), genetically undefined SCA, FRDA, and FRDA carriers to identify interruptions within the circuitry: calbindin-D28k, neuron-specific enolase, glutamic acid decarboxylase, and vesicular glutamate transporters 1 and 2. Lesions of the cerebellar cortex, DN, and ION were scored according to a guide as 0 (normal), 1 (mild), 2 (moderate), and 3 (severe). Results of each of the five immunohistochemical stains were examined separately for each of the three regions. Combining scores of each anatomical region and each stain yielded a total score as an indicator of pathological severity. Total scores ranged from 16 to 38 in SCA-1 (nine cases); 22 to 39 in SCA-2 (six cases); 9 to 15 in SCA-3 (four cases); and 13 and 25 in SCA-6 (two cases). In single cases of SCA-7 and SCA-17, scores were 16 and 31, respectively. In two genetically undefined SCA, scores were 36 and 37, respectively. In nine cases of FRDA, total scores ranged from 11 to 19. The low scores in SCA-3 and FRDA reflect selective atrophy of the DN. The FRDA carriers did not differ from normal controls. These observations offer a semiquantitative assessment of the critical role of the DN in the ataxic phenotype of SCA and FRDA while other parts of the circuitry appear less important.







Similar content being viewed by others
References
Uusisaari M, Knöpfel T. GlyT2+ neurons in the lateral cerebellar nucleus. Cerebellum. 2010;9:42–55.
Schaffer K. Gibt es eine cerebello-olivare Bahn? Zeitsch Ges Neurol Psychiat. 1915;30:70–83.
Guillain G, Mollaret P. Deux cas de myoclonies synchrones et rythmées vélo-pharyngo-laryngo-oculo-diaphragmatiques: Le problème anatomique et physiologique. Rev Neurol. 1931;2:545–66.
Lapresle J, Ben Hamida M. The dentato-olivary pathway. Somatotopic relationship between the dentate nucleus and the contralateral olive. Arch Neurol. 1970;22:135–43.
De Zeeuw CI, Holstege JC, Ruigrok TJH, Voogd J. Ultrastructural study of the GABAergic, cerebellar, and mesodiencephalic innervation of the cat medial accessory olive: Anterograde tracing combined with immunocytochemistry. J Comp Neurol. 1989;284:12–35.
Fredette BJ, Mugnaini E. The GABAergic cerebello-olivary projection in the rat. Anat Embryol. 1991;184:225–43.
Koeppen AH, Davis AN, Morral JA. The cerebellar component of Friedreich’s ataxia. Acta Neuropathol. 2011;122:323–30.
Ruigrok TJH. Ins and outs of cerebellar modules. Cerebellum. 2011;10:464–74.
Voogd J. Cerebellum and precerebellar nuclei. In: Paxinos G, Mai JK, editors. The human nervous system. 2nd ed. Amsterdam: Elsevier; 2004. p. 321–92.
Koeppen AH. Neuropathology of the inherited ataxias. In: Manto MU, Pandolfo M, editors. The cerebellum and its disorders. Cambridge: Cambridge University Press; 2002. p. 387–405.
Holmes G, Stewart T. On the connection of the inferior olives with the cerebellum in man. Brain. 1908;31:125–37.
Koeppen AH, Dickson AC, Lamarche JB, Robitaille Y. Synapses in the hereditary ataxias. J Neuropathol Exp Neurol. 1999;58:748–64.
Koeppen AH. Friedreich’s ataxia: pathology, pathogenesis, and molecular genetics. J Neurol Sci. 2011;303:1–12.
De Zeeuw CI, Simpson JI, Hoogenraad C, Galjart N, Koekkoek SKE, Ruigrok TJH. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400.
Llinás R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–71.
Heckroth JA, Eisenman LM. Olivary morphology and olivocerebellar topography in adult lurcher mutant mice. J Comp Neurol. 1991;312:641–51.
Uusisaari M, Knöpfel T. Functional classification of neurons in the mouse lateral cerebellar nuclei. Cerebellum. 2011;10:637–46.
Zafra F, Aragón C, Olivares L, Danbolt NC, Giménez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–69.
Pijpers A, Apps A, Pardoe J, Voogd J, Ruigrok TJH. Precise spatial relationship between mossy fibers and climbing fibers in rat cerebellar cortical zones. J Neurosci. 2006;26:12067–80.
Uusisaari M, Obata K, Knöpfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97:901–11.
Küper M, Thürling M, Maderwald S, Ladd ME, Timmann D. Structural and functional magnetic resonance imaging of the human cerebellar nuclei. Cerebellum. 2012;11:314–24.
Granziera C et al. Diffusion spectrum imaging shows the structural basis of functional cerebellar circuits in the human cerebellum in vivo. PLoS One. 2009;e5101:1–6.
Acknowledgments
The authors received financial support from the National Ataxia Foundation, Minneapolis, MN, USA; Friedreich’s Ataxia Research Alliance, Downingtown, PA, USA; Neurochemical Research, Inc., Glenmont, NY, USA; National Institutes of Health, Bethesda, MD, USA (grant no. R01-NS069454); and University of Tübingen, Tübingen, Germany. This work was performed in the research laboratories of the Veterans Affairs Medical Center, Albany, NY, USA. Dr. Rodney D. McComb, Omaha, NE, USA, contributed one case of FRDA. Sarah Collins provided expert technical assistance in immunohistochemistry.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Reciprocal cerebellar circuitry in SCA-7. The CAG trinucleotide repeats in this patient were 40 (expanded) and 10 (normal). The total score is 16, with the DN lesion accounting for 11 (68.8 %). The DN shows loss of NSE, GAD, and VGluT1 reaction product. Grumose degeneration is present after staining with anti-GAD (arrow). Abbreviations as in text Fig. 1. Bars: 50 μm (PPTX 1256 kb)
Supplemental Fig. 2
Reciprocal cerebellar circuitry in SCA-17. The CAG trinucleotide repeats in this patient were 55 (expanded) and (38 normal). The total score is 31, with the DN lesion accounting for 9 (29 %). Thinning of the ML (all stains) and lack of large neurons in the DN are prominent. Despite severe loss of Purkinje cells (calbindin-D28k/molecular layer), degeneration of the ION is moderate. The globular VGluT2 reaction product in the molecular layer is an artifact. Abbreviations as in text Fig. 1. Bars: 50 μm (PPTX 1212 kb)
Supplemental Fig. 3
Reciprocal cerebellar circuitry in SCA due to an unknown mutation. The total score is 37, with the DN lesion accounting for 12 (32.4 %). The lesion in the DN is manifest by loss of calbindin-D28k-, GAD-, VGluT1-, and VGluT2-reactive terminals. Small neurons in the DN survive (NSE and GAD, arrows). The severe abnormalities in the molecular layer and ION are similar with those in SCA-2 (text Fig. 3). Abbreviations as in text Fig. 1. Bars: 50 μm (PPTX 1144 kb)
Supplemental Fig. 4
Reciprocal cerebellar circuitry in an FRDA carrier. The GAA trinucleotide repeats in this patient were 845 (expanded) and 30 (normal). All antibodies yield normal immunohistochemical reaction products. Abbreviations as in text Fig. 1. Bars: 50 μm (PPTX 1282 kb)
Rights and permissions
About this article
Cite this article
Koeppen, A.H., Ramirez, R.L., Bjork, S.T. et al. The Reciprocal Cerebellar Circuitry in Human Hereditary Ataxia. Cerebellum 12, 493–503 (2013). https://doi.org/10.1007/s12311-013-0456-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-013-0456-0