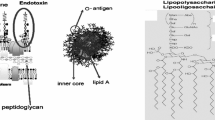
Abstract
Host defense against many invading Gram-negative bacteria (GNB) depends on innate immune recognition of endotoxin (lipopolysaccharides, LPS), unique surface glycolipids of GNB. Host responses to endotoxin must be highly sensitive but self-limited. In mammals, optimal sensitivity is achieved by ordered interactions of endotoxin with several different extracellular and cell surface proteins—the LPS-binding protein (LBP), CD14, MD-2, and Toll-like receptor (TLR) 4—reflecting the requirement for specific protein–endotoxin and protein–protein interactions. This complex reaction pathway also provides many ways to attenuate endotoxin-driven inflammation and can explain how differences in endotoxin structure, either intrinsic among GNB or induced by metabolic remodeling, can alter host responsiveness and thus the outcome of host-GNB interactions. Major goals of our research are to better understand: (1) the structural bases of specific host-endotoxin interactions; (2) functional diversity among host endotoxin-binding proteins; and (3) how the actions of various endotoxin-binding proteins are regulated to permit optimal host responses to GNB infection. In addition, the identification of a water-soluble endotoxin:MD-2 complex that, depending on the structure of endotoxin or MD-2, has potent TLR4 agonist or antagonist properties suggests novel pharmacologic approaches to immuno-modulation.




Similar content being viewed by others
References
Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol 2003;3:169–76.
Beutler B, Jiang Z, Georgel Z, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: toll-like receptor signaling and immunity at large. Annu Rev Immunol 2006;24:353–89.
Ulevitch RJ, Tobias PS. Recognition of Gram-negative bacteria and endotoxin by the innate immune system. Curr Opin Immunol 1999;11:19–24.
Alexander C, Rietschel ET. Bacterial lipopolysaccharides and innate immunity. J Endotoxin Res 2001;7:167–202.
Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta 2002;323:59–72.
Miyake K. Innate recognition of lipopolysaccharide by Toll-like receptor 4-MD-2. Trends Microbiol 2004;12:186–92.
Munford RS. Detoxifying endotoxin: time, place and person. J Endotoxin Res 2005;11:69–94.
Brandenburg K, Wiese A. Endotoxins: relationships between structure, function, and activity. Curr Top Med Chem 2004;4:1127–46.
Caroff M, Karibian D, Cavaillon JM, Haeffner-Cavaillon N. Structural and functional analyses of bacterial lipopolysaccharides. Microbes Infect 2002;4:915–24.
Gioannini TL, Teghanemt A, Zhang D, Levis EN, Weiss JP. Monomeric endotoxin: protein complexes are essential for TLR4-dependent cell activation. J Endotoxin Res 2005;11:117–23.
Miller SI, Ernst RK, Bader MW. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol 2005;3:36–46.
Giardina PC, Gioannini T, Buscher BA, Zaleski A, Zheng DS, Stoll L, Teghanemt A, Apicella MA, Weiss J. Construction of acetate auxotrophs of Neisseria meningitidis to study host-meningococcal endotoxin interactions. J Biol Chem 2001;276:5883–91.
Gioannini TL, Teghanemt A, Zarember KA, Weiss JP. Regulation of interactions of endotoxin with host cells. J Endotoxin Res 2003;9:401–9.
Dixon DR, Darveau RP. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid a structure. J Dental Res 2005;84:584–95.
Darveau RP. Lipid A diversity and the innate host response to bacterial infection. Curr Opin Microbiol 1998;1:36–42.
Post DMB, Zhang De S, Weiss JP, Gibson BW. Stable isotope metabolic labeling of Neisseria meningitidis lipooligosaccharide. J Endotoxin Res 2006;12:9–98.
Katz SS, Weinrauch Y, Munford RS, Elsbach P, Weiss J. Lipopolysaccharide deacylation following extracellular or intracellular killing of Escherichia coli by rabbit inflammatory peritoneal exudates. J Biol Chem 1999;274:36579–84.
Weinrauch Y, Elsbach P, Katz SS, Munford RS, Weiss J. Deacylation of purified lipopolysaccharide by cellular and extracellular components of sterile rabbit inflammatory exudates. Infect Immun 1999;67:3376–82.
Post DM, Zhang D, Eastvold JS, Teghanemt A, Gibson BW, Weiss JP. Biochemical and functional characterization of membrane blebs purified from Neisseria meningitidis serogroup B. J Biol Chem 2005;280:38383–91.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499–511.
Montiminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol 2006;7:1066–72.
Miyake K. Innate recognition of lipopolysaccharide by CD14 and toll-like receptor 4-MD-2: unique roles for MD-2. Int Immunopharmacol 2003;3:119–27.
Gioannini TL, Teghanemt A, Zhang D, Coussens NP, Dockstader W, Ramaswamy S, Weiss JP. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. Proc Natl Acad Sci USA 2004;101:4186–91.
Prohinar P, Re F, Widstrom R, Zhang DS, Teghanemt A, Weiss JP, Gioannini TL. Specific high affinity interactions of monomeric endotoxin-protein complexes with Toll-like receptor 4 ectodomain. J Biol Chem 2007;282:1010–7.
Gioannini TL, Zhang D, Teghanemt A, Weiss JP. An essential role for albumin in the interaction of endotoxin with lipopolysaccharide-binding protein and sCD14 and resultant cell activation. J Biol Chem 2002;277:47818–25.
Gioannini TL, Teghanemt A, Zhang DS, Prohinar P, Levis EN, Munford RS, Weiss JP. Endotoxin binding proteins modulate the susceptibility of bacterial endotoxin to deacylation by acyloxyacyl hydrolase. J. Biol. Chem 2007;282:7877–84.
Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat Immunol 2005;6:565–71.
Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect 2004;6:1361–9.
Kim JI, Lee CJ, Jin MS, Lee CH, Paik SG, Lee H, Lee JO. Crystal structure of CD14 and its implications for lipopolysaccharide signaling. J Biol Chem 2005;280:11347–51.
Teghanemt A, Zhang D, Levis EN, Weiss JP, Gioannini TL. Molecular basis of reduced potency of underacylated endotoxins. J Immunol 2005;175:4669–76.
Gruber A, Mancek M, Wagner H, Kirschning CJ, Jerala R. Structural model of MD-2 and functional role of its basic amino acid clusters involved in cellular lipopolysaccharide recognition. J Biol Chem 2004;279:28475–82.
Gangloff M, Gay NJ. MD-2: the Toll ‘gatekeeper’ in endotoxin signalling. Trends Biochem Sci 2004;29:294–300.
Ichikawa S, Takai T, Inoue T, Yuuki T, Okumura Y, Ogura K, Inagaki F, Hatanaka H. NMR study on the major mite allergen Der f 2: its refined tertiary structure, epitopes for monoclonal antibodies and characteristics shared by ML protein group members. J Biochem (Tokyo) 2005;137:255–63.
Wright CS, Li SC, Rastinejad F. Crystal structure of human GM2-activator protein with a novel beta-cup topology. J Mol Biol 2000;304:411–22.
Wright CS, Mi LZ, Lee S, Rastinejad F. Crystal structure analysis of phosphatidylcholine-GM2-activator product complexes: evidence for hydrolase activity. Biochemistry 2005; 44:13510–21.
Coats SR, Pham TT, Bainbridge BW, Reife RA, Darveau RP. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J Immunol 2005;175:4490–8.
Barker JH, Weiss J, Apicella MA, Nauseef WM. Basis for the failure of Francisella tularensis lipopolysaccharide to prime human polymorphonuclear leukocytes. Infect Immun 2006;74:3277–84.
Lamping N, Dettmer R, Schroder NW, Pfeil D, Hallatschek W, Burger R, Schumann RR. LPS-binding protein protects mice from septic shock caused by LPS or Gram-negative bacteria. J Clin Invest 1998;101:2065–71.
Kitchens RL, Thompson PA. Impact of sepsis-induced changes in plasma on LPS interactions with monocytes and plasma lipoproteins: roles of soluble CD14, LBP, and acute phase lipoproteins. J Endotoxin Res 2003;9:113–8.
Jia HP, Kline JN, Penisten A, Apicella MA, Gioannini TL, Weiss J, McCray PB Jr. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol 2004;287:L428–37.
Pugin J, Stern-Voeffray S, Daubeuf B, Matthay MA, Elson G, Dunn-Siegrist I. Soluble MD-2 activity in plasma from patients with severe sepsis and septic shock. Blood 2004;104:4071–9.
Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 2001;167:1609–16.
Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 2004;126:1054–70.
Weiss J. Bactericidal/permeability-increasing protein (BPI) and lipopolysaccharide-binding protein (LBP): structure, function and regulation in host defence against Gram-negative bacteria. Biochem Soc Trans 2003;31:785–91.
Dunzendorfer S, Lee HK, Soldau K, Tobias PS. TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J Immunol 2004;173:1166–70.
Stamme C, Muller M, Hamann L, Gutsmann T, Seydel U. Surfactant protein a inhibits lipopolysaccharide-induced immune cell activation by preventing the interaction of lipopolysaccharide with lipopolysaccharide-binding protein. Am J Respir Cell Mol Biol 2002;27:353–60.
Gioannini TL, Teghanemt A, Zarember KA, Weiss JP. Regulation of interactions of endotoxin with host cells. J Endotoxin Res 2003;9:401–9.
Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect 2006;8:946–52.
Hamann L, Alexander C, Stamme C, Zahringer U, Schumann RR. Acute-phase concentrations of lipopolysaccharide (LPS)-binding protein inhibit innate immune cell activation by different LPS chemotypes via different mechanisms. Infect Immun 2005;73:193–200.
Iovine N, Elsbach P, Weiss J. An opsonic function of the neutrophil bactericidal/permeability-increasing protein depends on both its N- and C-terminal domains. Proc Natl Acad Sci USA 1997;94:10973–78.
Iovine N, Eastvold J, Elsbach P, Weiss JP, Gioannini TL. The carboxyl-terminal domain of closely related endotoxin-binding proteins determines the target of protein-lipopolysaccharide complexes. J Biol Chem 2002;277:7970–8.
Mueller-Anneling L, Avol E, Peters JM, Thorne PS. Ambient endotoxin concentrations in PM10 from Southern California. Environ Health Perspect 2004;112:583–8.
Douwes J, Thorne P, Pearce N, Heederik D. Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg 2003;47:187–200.
Hollingsworth JW, Chen BJ, Brass DM, Berman K, Gunn MD, Cook DN, Schwartz DA. The critical role of hematopoietic cells in lipopolysaccharide-induced airway inflammation. Am J Respir Crit Care Med 2005;171:806–13.
Cluff CW, Baldridge JR, Stover AG, Evans JT, Johnson DA, Lacy MJ, Clawson VG, Yorgensen VM, Johnson CL, Livesay MT, Hershberg RM, Persing DH. Synthetic Toll-like receptor 4 agonists stimulate innate resistance to infection. Infect Immun 2005;73:3044–52.
Persing DH, Coler RN, Lacy MJ, Johnson DA, Baldridge JR, Hershberg RM, Reed SG. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol 2002;10:S32-7.
Fort MM, Mozaffarian A, Stover AG, Correia JdS, Johnson DA, Crane RT, Ulevitch RJ, Persing DH, Bielefeldt-Ohmann H, Probst P, Jeffery E, Fling SP, Hershberg RM. A synthetic TLR4 antagonist has anti-inflammatory effects in two murine models of inflammatory bowel disease. J Immunol 2005;174:6416–23.
Acknowledgments
We are indebted to the contributions and insights of Athmane Teghanemt, DeSheng Zhang, Polonca Prohinar, Deborah Post, Brad Gibson, Hong Peng Jia Paul McCray, Suzana Hadina, Peter Thorne, Nicole Iovine, Janet Hume, and Hendrik Schultz. Portions of the work reviewed here were supported by United States Public Health Service Grants PO144642 and AI59372 (to J.P.W.) and a Veterans’ Affairs Merit Review Grant (to T.L.G.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gioannini, T.L., Weiss, J.P. Regulation of interactions of Gram-negative bacterial endotoxins with mammalian cells. Immunol Res 39, 249–260 (2007). https://doi.org/10.1007/s12026-007-0069-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-007-0069-0