Abstract
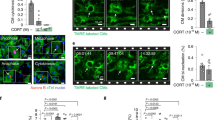
Glucocorticoids (GCs) are frequently prescribed pharmacological agents most notably for their immunosuppressive effects. Endogenous GCs mediate biological processes such as energy metabolism and tissue development. At the cellular level, GCs bind to the glucocorticoid receptor (GR), a cytosolic protein that translocates to the nuclei and functions to alter transcription upon ligand binding. Among a long list of genes activated by GCs is the glucocorticoid-induced leucine zipper (GILZ). GC-induced GILZ expression has been well established in lymphocytes and mediates GC-induced apoptosis. Unlike lymphocytes, cardiomyocytes respond to GCs by gaining resistance against apoptosis. We determined GILZ expression in cardiomyocytes in vivo and in vitro. Expression of GILZ in mouse hearts as a result of GC administration was confirmed by Western blot analyses. GCs induced dose- and time-dependent elevation of GILZ expression in primary cultured rat cardiomyocytes, with dexamethasone (Dex) as low as 0.1 μM being effective. Time course analysis indicated that GILZ protein levels increased at 8 h and peaked at 48 h after exposure to 1 μM Dex. H9c2(2-1) cell line showed a similar response of GILZ induction by Dex as primary cultured rat cardiomyocytes, providing a convenient model for studying the biological significance of GILZ expression. With corticosterone (CT), an endogenous form of corticosteroids in rodents, 0.1–2.5 μM was found to induce GILZ in H9c2(2-1) cells. Time course analysis with 1 μM CT indicated induction of GILZ at 6 h with peak expression at 18 h. Inhibition of the GR by mifepristone led to blunting of GILZ induction by GCs. Our data demonstrate GILZ induction in cardiomyocytes both in vivo and in vitro by GCs, pointing to H9c2(2-1) cells as a valid model for studying the biological function of GILZ in cardiomyocytes.








Similar content being viewed by others
References
D’Adamio, F., Zollo, O., Moraca, R., Ayroldi, E., Bruscoli, S., Bartoli, A., et al. (1997). A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity, 7, 803–812.
Ayroldi, E., Migliorati, G., Bruscoli, S., Marchetti, C., Zollo, O., Cannarile, L., et al. (2001). Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood, 98, 743–753.
Ayroldi, E., Zollo, O., Bastianelli, A., Marchetti, C., Agostini, M., Di Virgilio, R., et al. (2007). GILZ mediates the antiproliferative activity of glucocorticoids by negative regulation of Ras signaling. Journal of Clinical Investment, 117, 1605–1615.
Muller, O. G., Parnova, R. G., Centeno, G., Rossier, B. C., Firsov, D., & Horisberger, J. D. (2003). Mineralocorticoid effects in the kidney: Correlation between alphaENaC, GILZ, and Sgk-1 mRNA expression and urinary excretion of Na+ and K+. Journal of the American Society of Nephrology, 14, 1107–1115.
Soundararajan, R., Zhang, T. T., Wang, J., Vandewalle, A., & Pearce, D. (2005). A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. Journal of Biological Chemistry, 280, 39970–39981.
Di Marco, B., Massetti, M., Bruscoli, S., Macchiarulo, A., Di Virgilio, R., Velardi, E., et al. (2007). Glucocorticoid-induced leucine zipper (GILZ)/NF-kappaB interaction: Role of GILZ homo-dimerization and C-terminal domain. Nucleic Acids Research, 35, 517–528.
Mittelstadt, P. R., & Ashwell, J. D. (2001). Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. Journal of Biological Chemistry, 276, 29603–29610.
Ayroldi, E., Zollo, O., Macchiarulo, A., Di Marco, B., Marchetti, C., & Riccardi, C. (2002). Glucocorticoid-induced leucine zipper inhibits the Raf-extracellular signal-regulated kinase pathway by binding to Raf-1. Molecular and Cellular Biology, 22, 7929–7941.
Ayroldi, E., & Riccardi, C. (2009). Glucocorticoid-induced leucine zipper (GILZ): A new important mediator of glucocorticoid action. FASEB Journal, 23, 3649–3658.
Cannarile, L., Zollo, O., D’Adamio, F., Ayroldi, E., Marchetti, C., Tabilio, A., et al. (2001). Cloning, chromosomal assignment and tissue distribution of human GILZ, a glucocorticoid hormone-induced gene. Cell Death and Differentiation, 8, 201–203.
Asselin-Labat, M. L., David, M., Biola-Vidamment, A., Lecoeuche, D., Zennaro, M. C., Bertoglio, J., et al. (2004). GILZ, a new target for the transcription factor FoxO3, protects T lymphocytes from interleukin-2 withdrawal-induced apoptosis. Blood, 104, 215–223.
Tynan, S. H., Lundeen, S. G., & Allan, G. F. (2004). Cell type-specific bidirectional regulation of the glucocorticoid-induced leucine zipper (GILZ) gene by estrogen. Journal of Steroid Biochemistry and Molecular Biology, 91, 225–239.
Chen, Q. M., Alexander, D., Sun, H., Xie, L., Lin, Y., Terrand, J., et al. (2005). Corticosteroids inhibit cell death induced by doxorubicin in cardiomyocytes: Induction of antiapoptosis, antioxidant, and detoxification genes. Molecular Pharmacology, 67, 1861–1873.
Gerrelli, D., Huntriss, J. D., & Latchman, D. S. (1994). Antagonistic effects of retinoic acid and thyroid hormone on the expression of the tissue-specific splicing protein SmN in a clonal cell line derived from rat heart. Journal of Molecular and Cellular Cardiology, 26, 713–719.
Khaw, B. A., Torchilin, V. P., Vural, I., & Narula, J. (1995). Plug and seal: Prevention of hypoxic cardiocyte death by sealing membrane lesions with antimyosin-liposomes. Nature Medicine, 1, 1195–1198.
Mestril, R., Chi, S. H., Sayen, M. R., O’Reilly, K., & Dillmann, W. H. (1994). Expression of inducible stress protein 70 in rat heart myogenic cells confers protection against simulated ischemia-induced injury. Journal of Clinical Investment, 93, 759–767.
Coronella-Wood, J., Terrand, J., Sun, H., & Chen, Q. M. (2004). c-Fos phosphorylation induced by H2O2 prevents proteasomal degradation of c-Fos in cardiomyocytes. Journal of Biological Chemistry, 279, 33567–33574.
Xu, B., Strom, J., & Chen, Q. M. (2011). Dexamethasone induces transcriptional activation of Bcl-xL gene and inhibits cardiac injury by myocardial ischemia. European Journal of Pharmacology, 668, 194–200.
Schmidt, P., Holsboer, F., & Spengler, D. (2001). Beta(2)-adrenergic receptors potentiate glucocorticoid receptor transactivation via G protein beta gamma-subunits and the phosphoinositide 3-kinase pathway. Molecular Endocrinology, 15, 553–564.
Hafezi-Moghadam, A., Simoncini, T., Yang, Z., Limbourg, F. P., Plumier, J. C., Rebsamen, M. C., et al. (2002). Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nature Medicine, 8, 473–479.
Harms, C., Albrecht, K., Harms, U., Seidel, K., Hauck, L., Baldinger, T., et al. (2007). Phosphatidylinositol 3-Akt-kinase-dependent phosphorylation of p21(Waf1/Cip1) as a novel mechanism of neuroprotection by glucocorticoids. Journal of Neuroscience, 27, 4562–4571.
Hulley, P. A., Gordon, F., & Hough, F. S. (1998). Inhibition of mitogen-activated protein kinase activity and proliferation of an early osteoblast cell line (MBA 15.4) by dexamethasone: role of protein phosphatases. Endocrinology, 139, 2423–2431.
Gonzalez, M. V., Gonzalez-Sancho, J. M., Caelles, C., Munoz, A., & Jimenez, B. (1999). Hormone-activated nuclear receptors inhibit the stimulation of the JNK and ERK signalling pathways in endothelial cells. FEBS Letters, 459, 272–276.
Kassel, O., Sancono, A., Kratzschmar, J., Kreft, B., Stassen, M., & Cato, A. C. (2001). Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO Journal, 20, 7108–7116.
Lasa, M., Abraham, S. M., Boucheron, C., Saklatvala, J., & Clark, A. R. (2002). Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Molecular and Cellular Biology, 22, 7802–7811.
Shuto, T., Imasato, A., Jono, H., Sakai, A., Xu, H., Watanabe, T., et al. (2002). Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. Journal of Biological Chemistry, 277, 17263–17270.
Kimes, B. W., & Brandt, B. L. (1976). Properties of a clonal muscle cell line from rat heart. Experimental Cell Research, 98, 367–381.
Hescheler, J., Meyer, R., Plant, S., Krautwurst, D., Rosenthal, W., & Schultz, G. (1991). Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circulation Research, 69, 1476–1486.
Sipido, K. R., & Marban, E. (1991). L-type calcium channels, potassium channels, and novel nonspecific cation channels in a clonal muscle cell line derived from embryonic rat ventricle. Circulation Research, 69, 1487–1499.
Watkins, S. J., Borthwick, G. M., & Arthur, H. M. (2011). The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Develop Biology. Animal, 47, 125–131.
Chen, Q. M., Tu, V. C., Wu, Y., & Bahl, J. J. (2000). Hydrogen peroxide dose dependent induction of cell death or hypertrophy in cardiomyocytes. Archives of Biochemistry and Biophysics, 373, 242–248.
Menconi, M., Gonnella, P., Petkova, V., Lecker, S., & Hasselgren, P. O. (2008). Dexamethasone and corticosterone induce similar, but not identical, muscle wasting responses in cultured L6 and C2C12 myotubes. Journal of Cellular Biochemistry, 105, 353–364.
Solito, E., Mulla, A., Morris, J. F., Christian, H. C., Flower, R. J., & Buckingham, J. C. (2003). Dexamethasone induces rapid serine-phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel nongenomic mechanism involving the glucocorticoid receptor, protein kinase C, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase. Endocrinology, 144, 1164–1174.
Limbourg, F. P., & Liao, J. K. (2003). Nontranscriptional actions of the glucocorticoid receptor. Journal of Molecular Medicine (Berlin), 81, 168–174.
Poizat, C., Puri, P. L., Bai, Y., & Kedes, L. (2005). Phosphorylation-dependent degradation of p300 by doxorubicin-activated p38 mitogen-activated protein kinase in cardiac cells. Molecular and Cellular Biology, 25, 2673–2687.
Asai, M., Tsukamoto, O., Minamino, T., Asanuma, H., Fujita, M., Asano, Y., et al. (2009). PKA rapidly enhances proteasome assembly and activity in in vivo canine hearts. Journal of Molecular and Cellular Cardiology, 46, 452–462.
Drews, O., Tsukamoto, O., Liem, D., Streicher, J., Wang, Y., & Ping, P. (2010). Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circulation Research, 107, 1094–1101.
Lin, A. L., McGill, H. C., Jr, & Shain, S. A. (1982). Hormone receptors of the baboon cardiovascular system. Biochemical characterization of aortic and myocardial cytoplasmic progesterone receptors. Circulation Research, 50, 610–616.
Goldstein, J., Sites, C. K., & Toth, M. J. (2004). Progesterone stimulates cardiac muscle protein synthesis via receptor-dependent pathway. Fertility and Sterility, 82, 430–436.
Grohe, C., Kahlert, S., Lobbert, K., Stimpel, M., Karas, R. H., Vetter, H., et al. (1997). Cardiac myocytes and fibroblasts contain functional estrogen receptors. FEBS Letters, 416, 107–112.
Ingegno, M. D., Money, S. R., Thelmo, W., Greene, G. L., Davidian, M., Jaffe, B. M., et al. (1988). Progesterone receptors in the human heart and great vessels. Laboratory Investigation, 59, 353–356.
Knowlton, A. A., & Sun, L. (2001). Heat-shock factor-1, steroid hormones, and regulation of heat-shock protein expression in the heart. American Journal of Physiolology Heart Circulation Physiology, 280, H455–H464.
Evans, R. M. (1988). The steroid and thyroid hormone receptor superfamily. Science, 240, 889–895.
Cato, A. C., Miksicek, R., Schutz, G., Arnemann, J., & Beato, M. (1986). The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO Journal, 5, 2237–2240.
Strahle, U., Klock, G., & Schutz, G. (1987). A DNA sequence of 15 base pairs is sufficient to mediate both glucocorticoid and progesterone induction of gene expression. Proceedings of the Natural Academy Sciences of USA, 84, 7871–7875.
Hecht, A., Berkenstam, A., Stromstedt, P. E., Gustafsson, J. A., & Sippel, A. E. (1988). A progesterone responsive element maps to the far upstream steroid dependent DNase hypersensitive site of chicken lysozyme chromatin. EMBO Journal, 7, 2063–2073.
Ham, J., Thomson, A., Needham, M., Webb, P., & Parker, M. (1988). Characterization of response elements for androgens, glucocorticoids and progestins in mouse mammary tumour virus. Nucleic Acids Research, 16, 5263–5276.
Gronemeyer, H. (1991). Transcription activation by estrogen and progesterone receptors. Annual Review Genetics, 25, 89–123.
Morrissy, S., Xu, B., Aguilar, D., Zhang, J., & Chen, Q. M. (2010). Inhibition of apoptosis by progesterone in cardiomyocytes. Aging Cell, 9, 799–809.
De, P., Roy, S. G., Kar, D., & Bandyopadhyay, A. (2011). Excess of glucocorticoid induces myocardial remodeling and alteration of calcium signaling in cardiomyocytes. Journal of Endocrinology, 209, 105–114.
Acknowledgments
Work from our laboratory has been supported by NIH R01 HL 076530, R01 HL089958, R21ES017473, T32 ES007091, Arizona Biomedical Research Commission (QMC). We would like to thank Daniel Lee for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguilar, D.C., Strom, J., Xu, B. et al. Expression of Glucocorticoid-Induced Leucine Zipper (GILZ) in Cardiomyocytes. Cardiovasc Toxicol 13, 91–99 (2013). https://doi.org/10.1007/s12012-012-9188-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-012-9188-5