Abstract
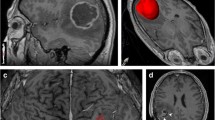
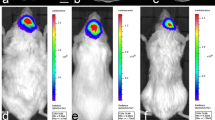
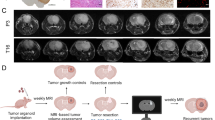
Distinguishing tumor progression from radiation necrosis after treatment in patients with brain tumors presents a clinical dilemma. A well-characterized, orthotopic rodent model of radiation-induced brain necrosis including a tumor is not currently available The objective of the study was to create focal radiation necrosis in rat brain bearing human glioblastoma (GBM) using stereotactic radiosurgery and confirm it by immuno-histological analysis. Nude rats implanted with primary GBM cells were irradiated using a stereotactic setup (n = 3) or received no radiation (n = 3). Ten weeks after the implantation, growth of the tumor was confirmed by magnetic resonance imaging (MRI). For each animal, MRI and contrast-enhanced CT images were obtained and fused using registration software. The tumor was identified and delineated using the fused CT/MR images. A treatment plan was generated using a 4 mm radiosurgery cone such that one portion of the tumor receives 100% dose of 60 Gy sufficient to cause necrosis, whereas the tumor edge at depth receives only 50% or less dose, allowing for regrowth of the tumor. The brains were collected 10 weeks after irradiation and immuno-histological analysis was performed. Hematoxylin and eosin staining showed central liquefaction necrosis in the high dose region consistent with necrosis and viable tumor in the peripheral low dose region. Ki-67 staining showed highly proliferative tumor cells surrounding the necrotic parts of the tumor. Luxol fast blue and lectin staining showed demyelination and vascular injury in brain tissue consistent with radiation necrosis. We have developed a novel model of radiation necrosis in rats bearing glioma.









Similar content being viewed by others
References
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Macdonald DR, Cascino TL, Schold SC Jr et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Brandsma D, van den Bent MJ (2009) Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22(6):633–638
Clarke JL, Chang S (2009) Pseudoprogression and pseudoresponse: challenges in brain tumor imaging. Curr Neurol Neurosci Rep 9(3):241–246
Chamberlain MC, Glantz MJ, Chalmers L et al (2007) Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol 82(1):81–83
de Wit MC, de Bruin HG, Eijkenboom W et al (2004) Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 63(3):535–537
Chaskis C, Neyns B, Michotte A et al (2009) Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol 72(4):423–428
Sanghera P, Perry J, Sahgal A et al (2010) Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci 37(1):36–42
Ruben JD, Dally M, Bailey M et al (2006) Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 65:499–508
Emami B, Lyman J, Brown A et al (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21:109–122
Marks LB, Yorke ED, Jackson A et al (2010) Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 76:S10–S19
Jain R, Scarpace L, Ellika S et al (2007) First pass perfusion CT: initial experience in differentiating recurrent brain tumors from radiation effects/radiation necrosis. Neurosurgery 61(4):778–787
Hein PA, Eskey CJ, Dunn JF et al (2004) Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. Am J Neuroradiol 25:201–209
Rachinger W, Goetz C, Pöpperl G, Gildehaus FJ et al (2005) Positron emission tomography with O-(2-[18F] fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57(3):505–511
Tsuyuguchi N, Takami T, Sunada I et al (2004) Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery—in malignant glioma. Ann Nucl Med 18(4):291–296
Kumar AJ, Leeds NE, Fuller GN et al (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384
Mullins ME, Barest GD, Schaefer PW et al (2005) Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. Am J Neuroradiol 26:1967–1972
Barth RF (1998) Rat brain tumor models in experimental neuro-oncology: the 9L, C6, T9, F98, RG2 (D74), RT-2 and CNS-1 gliomas. J Neurooncol 36(1):91–102
Zhou J, Tryggestad E, Wen Z et al (2011) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17(1):130–134
Kondziolka D, Lunsford LD, Claassen D et al (1992) Radiobiology of radiosurgery: Part II. The rat C6 glioma model. Neurosurgery 31(2):280–287
Jost SC, Hope A, Kiehl E et al (2009) A novel murine model for localized radiation necrosis and its characterization using advanced magnetic resonance imaging. Int J Radiat Oncol Biol Phys 75(2):527–533
deCarvalho AC, Nelson K, Lemke N et al (2010) Gliosarcoma stem cells undergo glial and mesenchymal differentiation in vivo. Stem Cells 28(2):181–190
Arbab AS, Janic B, Jafari-Khouzani K et al (2010) Differentiation of glioma and radiation injury in rats using in vitro produce magnetically labeled cytotoxic T-cells and MRI. PLoS One 5(2):e9365
Schultheiss TE, Kun LE, Ang KK et al (1995) Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 31:1093–1112
Postma TJ, Klein M, Verstappen CC et al (2002) Radiotherapy-induced cerebral abnormalities in patients with low-grade glioma. Neurology 59:121–123
Constine LS, Konski A, Ekholm S et al (1998) Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys 15:319–330
Tsuruda JS, Kortman KE, Bradley WG et al (1987) Radiation effects on cerebral white matter: MR evaluation. Am J Roentgenol 49:165–171
Wong CS, Van der Kogel AJ (2004) Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv 4:273–284
Belka C, Budach W, Kortmann RD et al (2001) Radiation induced CNS toxicity: molecular and cellular mechanisms. Br J Cancer 85:1233–1239
Fike JR, Sheline GE, Cann CE et al (1984) Radiation necrosis. Prog Exp Tumor Res 28:136–151
Tofilon PJ, Fike JR (2000) The radioresponse of the central nervous system: a dynamic process. Radiat Res 153:357–370
Yamaguchi N, Yamashima T, Yamashita J (1991) A histological and flow cytometric study of dog brain endothelial cell injuries in delayed radiation necrosis. J Neurosurg 74(4):625–632
Lunsford LD, Altschuler EM, Flickinger JC et al (1990) In vivo biological effects of stereotactic radiosurgery: a primate model. Neurosurgery 27(3):373–382
Kondziolka D, Lunsford LD, Claassen D et al (1992) Radiobiology of radiosurgery: Part I. The normal rat brain model. Neurosurgery 31(2):271–279
Spiegelmann R, Friedman WA, Bova FJ et al (1993) LINAC radiosurgery: an animal model. J Neurosurg 78(4):638–644
Tiller-Borcich JK, Fike JR et al (1987) Pathology of delayed radiation brain damage: an experimental canine model. Radiat Res 110(2):161–172
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S., Arbab, A.S., Jain, R. et al. Development of a novel animal model to differentiate radiation necrosis from tumor recurrence. J Neurooncol 108, 411–420 (2012). https://doi.org/10.1007/s11060-012-0846-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0846-z