Abstract
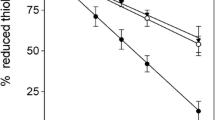
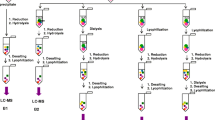
Formation of lanthionine, a dehydroalanine crosslink, is associated with aging of the human lens and cataractogenesis. In this study we investigated whether modification of lens proteins by glutathione could proceed through an alternative pathway: that is, by the formation of a nonreducible thioether bond between protein and glutathione. Direct ELISA of the reduced water-soluble and water-insoluble lens proteins from human cataractous, aged and bovine lenses showed a concentration-dependent immunoreactivity toward human nonreducible glutathionyl-lens proteins only. The reduced water-insoluble cataractous lens proteins showed the highest immunoreactivity, while bovine lens protein exhibited no reaction. These data were confirmed by dot-blot analysis. The level of this modification ranged from 0.7 to 1.6 nmol/mg protein in water-insoluble proteins from aged and cataractous lenses. N-terminal amino acid determination in the reduced and alkylated lens proteins, performed by derivatization of these preparations with dansyl chloride followed by an exhaustive dialysis, acid hydrolysis and fluorescence detection of dansylated amino acids by RP-HPLC, showed that N-terminal glutamic acid was present in concentration of approximately 0.2 nmol/mg of lens protein. This evidence points out that at least some of the N-terminal amino groups of nonreducible glutathione in the reduced human lens proteins are not involved in a covalent bond formation. Since disulfides were not detected in the reduced and alkylated human lens proteins, GSH is most likely attached to lens proteins through thioether bonds. These results provide, for the first time, evidence that glutathiolation of human lens proteins can occur through the formation of nonreducible thioether bonds.
Similar content being viewed by others
References
Masters PM, Bada JL, Zigler JS: Aspartic acid racemization in heavy molecular weight crystallins and water-insoluble protein from normal human lenses and cataracts. PNAS 75: 1204–1208, 1978
Kuboki Y, Fujisawa R, Tsuzaki M, Liu CF, Sasaki S: Presence of lysinoalanine and histidinoalanine in bovine dentin phosphoprotein. Calc Tissue Int 36: 126–128, 1984
Luthra M, Ranganathan D, Ranganathan S, Balasubramanian, D: Racemization of tyrosine in the insoluble protein fraction of brunescent aging human lenses. J Biol Chem 269: 22678–22682, 1994
Fujii N, Momose Y, Ishii N, et al.: The mechanisms of simultaneous stereoinversion, racemization, and isomerization at specific aspartyl residues of aged lens proteins. Mech Aging Dev 107: 347–358, 1999
Friedman M: Chemistry, biochemistry, nutrition, and microbiology of lysinoalanine, lanthionine, and histidinoalanine in food, and other proteins. J Agric Food Chem 47: 1295–1319, 1999
Finley JW, Friedman M: New amino acid derivatives formed by alkaline treatment of proteins. Adv Exp Med Biol 86(B): 123–130, 1977
Friedman M, Finley JW, Yeh LS: Reactions of proteins with dehydroalanines. Adv Exp Med Biol 86(B): 213–224, 1977
Cloos PAC, Jensen AL: Age-related de-phosphorylation of proteins in dentin: A biological tool for assessment of protein age. Biogerontology 1: 341–356, 2000
Kanayama T, Miyanaga Y, Horiuchi K, Fujimoto D: Detection of the cross-linking amino acid, histidinoalanine, in human brown cataractous lens protein. Exp Eye Res 44: 165–169, 1987
Bessems GJ, Rennen HJ, Hoenders HJ: Lanthionine, a protein cross-link in cataractous human lenses. Exp Eye Res 44: 691–695, 1987
Linetsky M, Hill JW, LeGrand RD, Hu F: Dehydroalanine crosslinks in human lens. Exp Eye Res 79: 499–512, 2004
Srivastava OP, Kirk MC, Srivastava K: Characterization of covalent multimers of crystallins in aging human lenses. J Biol Chem 279: 10901–10909, 2004
Kamei A: Glutathione levels of the human crystalline lens in aging and its antioxidant effect against the oxidation of lens proteins. Biol Pharm Bull 116: 870–875, 1993
Amara A, Coussemacq M, Geffard M: Antibodies to reduced glutathione. Brain Res 659: 237–242, 1994
Tabachnick M, Sobotka H: Azoproteins. A spectrophotometric study of the coupling of diazotized arsanilic acid with proteins. J Biol Chem 235: 1051–1054, 1960
Nashef AS, Osuga DT, Lee HS, Ahmed AI, Whitaker JR, Feeney RE: Effect of alkali on proteins. Disulfides and their products. J Agric Food Chem 25: 245–251, 1977
Kondo T, Kondo Y, Ui N: Colorimetry of dehydroalanine residues preserved as ‘lost side chains’ in thyroglobulin. Mol Cell Endocrinol 57: 101–106, 1988
Levina NB, Nazimov IV: High-performance liquid chromatography of Dns-amino acids in the purity control of peptides. J Chromatogr ([0-9]+): 207–216, 1984
Ellman GL: Tissue sulfhydryl groups. Arch Biochem Biophys 82: 70–77, 1959
Riddles PW, Blakeley RL, Zerner B: Ellman’s reagent: 5,5′-Dithiobis(2-nitrobenzoic acid) – a reexamination. Anal Biochem 94: 75–81, 1979
Snyder SL, Sobocinski PZ: An improved 2,4,6-dinitrobenzenesulfonic acid method for the determination of amines. Anal Biochem 64: 284–288, 1975
Kumari K, Khanna P, Ansari NH, Srivastava SK: High-performance liquid chromatography method for the determination of protein–glutathione disulfide. Anal Biochem 220: 374–376, 1994
Meyer HE, Heber M, Eisermann B, et al.: Sequence analysis of lantibiotics: Chemical derivatization procedures allow a fast access to complete Edman degradation. Anal Biochem 223: 185–190, 1994
Lou MF, Huang QL, Zigler JS Jr: Effect of opacification and pigmentation on human lens protein thiol/disulfide and solubility. Curr Eye Res 8: 883–890, 1989
Graghill J, Cronshaw AD, Harding JJ: The identification of a reaction site of glutathione mixed disulfide formation on γS-crystallin in human lens. Biochem J 379: 595–600, 2004
Nagaraj RH, Portero-Otin M, Monnier VM: Pyrraline ether crosslinks as a basis for protein crosslinking by the advanced Maillard reaction in aging and diabetes. Arch Biochem Biophys 325: 152–158, 1996
Marion MS, Carlson EC: Immunoelectron microscopic analyses of Maillard reaction products in bovine anterior lens capsule and Descemet’s membrane. Biochim Biophys Acta 1191: 33–42, 1994
Nagaraj RH, Sady C: The presence of a glucose-derived Maillard reaction product in the human lens. FEBS Lett 382: 234–238, 1996
Rose NL, Palcic MM, Sporns P, McMullen LM: Nisin: A novel substrate for glutathione S-transferase isolated from fresh beef. J Food Sci 2288–2293, 2002
Rose NL, Sporns P, Dodd HM, et al.: Involvement of dehydroalanine and dehydrobutyrine in the addition of glutathione to nisin. J Agric Food Chem 51: 3174–3178, 2003
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linetsky, M., LeGrand, R.D. Glutathionylation of lens proteins through the formation of thioether bond. Mol Cell Biochem 272, 133–144 (2005). https://doi.org/10.1007/s11010-005-6908-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11010-005-6908-1