Abstract
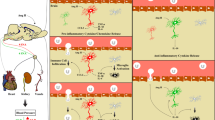
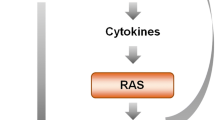
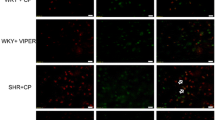
In addition to regulating blood pressure, Angiotensin II (Ang II) exerts powerful pro-inflammatory effects in hypertension through stimulation of its AT1 receptors, most clearly demonstrated in peripheral arteries and in the cerebral vasculature. Administration of Ang II receptor blockers (ARBs) decreases hypertension-related vascular inflammation in peripheral organs. In rodent models of genetic hypertension, ARBs reverse the inflammation in the cerebral microcirculation. We hypothesized that ARBs could be effective in inflammatory conditions beyond hypertension. Our more recent studies, summarized here, indicate that this is indeed the case. We used the model of systemic administration of the bacterial endotoxin lipopolysaccharide (LPS). LPS produces a robust initial inflammatory reaction, the innate immune response, in peripheral organs and in the brain. Pretreatment with the ARB candesartan significantly diminishes the response to LPS, including reduction of pro-inflammatory cytokine release to the general circulation and decreased production and release of the pro-inflammatory adrenal hormone aldosterone. In addition, the ARB very significantly decreased the LPS-induced gene expression of pro-inflammatory cytokines and microglia activation in the brain. Our results demonstrate that AT1 receptor activity is essential for the unrestricted development of full-scale innate immune response in the periphery and in the brain. ARBs, due to their immune response-limiting properties, may be considered as therapeutically useful in a number of inflammatory diseases of the peripheral organs and the brain.





Similar content being viewed by others
Abbreviations
- Ang II:
-
Angiotensin II
- AT1 :
-
Angiotensin II receptor type 1
- COX-2:
-
Cyclooxygenase 2
- ICAM-1:
-
Intercellular adhesion molecule 1
- IκBα:
-
Nuclear factor of kappa-light polypeptide gene enhancer in B-cells inhibitor, alpha
- IL-1β:
-
Interleukin 1β
- IL-6:
-
Interleukin 6
- iNOS:
-
Inducible nitric oxide synthase
- LBP:
-
LPS binding protein
- MAPKs:
-
Mitogen-activated protein kinases
- MCP-1:
-
Monocyte chemotactic protein-1
- NFκB:
-
Nuclear factor κB
- PGE2 :
-
Prostaglandin E2
- PLA2 :
-
Phospholipase A2
- ROS:
-
Reactive oxygen species
- sCD14:
-
Soluble CD14
- TLR-4:
-
Toll-like receptor 4
- TNF-α:
-
Tumor necrosis factor alpha
- VCAM-1:
-
Vascular cell adhesion molecule 1
References
Ando H, Zhou J, Macova M, Imboden H, Saavedra JM (2004) Angiotensin II AT1 receptor blockade reverses pathological hypertrophy and inflammation in brain microvessels of spontaneously hypertensive rats. Stroke 35:1726–1731. doi:10.1161/01.STR.0000129788.26346.18
Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terrón JA, Falcón-Neri A, Ito T, Juorio AV, Saavedra JM (2001) Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology 142:3880–3889. doi:10.1210/en.142.9.3880
Armando I, Volpi S, Aguilera G, Saavedra JM (2007) Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res 1142:92–99. doi:10.1016/j.brainres.2007.01.037
Basile JN, Chrysant S (2006) The importance of early antihypertensive efficacy: the role of angiotensin II receptor blocker therapy. J Hypertens 24(Suppl):S131–S137
Bosshart H, Heinzelmann M (2007) Targeting bacterial endotoxin: two sides of a coin. Ann N Y Acad Sci 1096:1–17. doi:10.1196/annals.1397.064
Bregonzio C, Armando I, Ando H, Jezova M, Baiardi G, Saavedra JM (2003) Anti-inflammatory effects of angiotensin II AT1 receptor antagonism prevent stress-induced gastric injury. Am J Physiol Gastrointest Liver Physiol 285:G414–G423
Bregonzio C, Seltzer A, Armando I, Pavel J, Saavedra JM (2008) Angiotensin II AT(1) receptor blockade selectively enhances brain AT(2) receptor expression, and abolishes the cold-restraint stress-induced increase in tyrosine hydroxylase mRNA in the locus coeruleus of spontaneously hypertensive rats. Stress 11:457–466
Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnette RM, Almeida OP, Davis TM (2008) Predictors of cognitive decline in older individuals with diabetes. Diabetes Care 31:2103–2107. doi:10.2337/dc08-0562
Cheng ZJ, Vapaatalo H, Mervaala E (2005) Angiotensin II and vascular inflammation. Med Sci Monit 11:RA194–RA205
Ching S, Zhang H, Belevych N, He L, Lai W, Pu X, Jaeger LB, Chen Q, Quan N (2007) Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci 27:10476–10486. doi:10.1523/JNEUROSCI.3357-07.2007
Connell JM, Davies E (2005) The new biology of aldosterone. J Endocrinol 186:1–20. doi:10.1677/joe.1.06017
Cover PO, Slater D, Buckingham JC (2001) Expression of cyclooxygenase enzymes in rat hypothalamo-pituitary-adrenal axis: effects of endotoxin and glucocorticoids. Endocrine 16:123–131. doi:10.1385/ENDO:16:2:123
Dahlöf B (2006) Prospects for the prevention of stroke. J Hypertens 24(Suppl):S3–S9
Dauphinee SM, Karsan A (2006) Lipopolysaccharide signaling in endothelial cells. Lab Invest 86:9–22. doi:10.1038/labinvest.3700366
Dong Z, Wei H, Sun R, Tian Z (2007) The roles of innate immune cells in liver injury and regeneration. Cell Mol Immunol 4:241–252
Dutta G, Zhang P, Liu B (2008) The lipopolysaccharide Parkinson’s disease animal model: mechanistic studies and drug discovery. Fund Clin Pharmacol 22:453–464
Fassbender K, Walter S, Kühl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, Rivest S, Lentschat A, Gulbins E, Jucker M, Staufenbiel M, Brechtel K, Walter J, Multhaup G, Penke B, Adachi Y, Hartmann T, Beyreuther K (2004) The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J 18:203–205
Garden GA, Moller T (2006) Microglia biology in health and disease. J Neuroimmune Pharmacol 1:127–137. doi:10.1007/s11481-006-9015-5
Gomez-Sanchez EP (2004) Brain mineralocorticoid receptors: orchestrators of hypertension and end-organ disease. Curr Opin Nephrol Hypertens 13:191–196. doi:10.1097/00041552-200403000-00007
Grinevich V, Ma XM, Herman JP, Jezova D, Akmayev I, Aguilera G (2001) Effect of repeated lipopolysaccharide administration on tissue cytokine expression and hypothalamic-pituitary-adrenal axis activity in rats. J Neuroendocrinol 13:711–723. doi:10.1046/j.1365-2826.2001.00684.x
Han J, Ulevitch RJ (2005) Limiting inflammatory responses during activation of innate immunity. Nat Immunol 6:1198–1205. doi:10.1038/ni1274
Henry CJ, Huang Y, Wynne AM, Godbout JP (2008) Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory Il-10 cytokines. Brain Behav Immun. doi:10.1016/j.bbi.2008.09-002
Hopkins SJ (2007) Central nervous system recognition of peripheral inflammation: a neural, hormonal collaboration. Acta Biomed 78(Suppl 1):231–247
Ichitani Y, Holmberg K, Maunsbach AB, Haeggstrom JZ, Samuelsson B, De Witt D, Hökfelt T (2001) Cyclooxygenase-1 and cyclooxygenase-2 expression in rat kidney and adrenal gland after stimulation with systemic lipopolysaccharide: in situ hybridization and immunocytochemical studies. Cell Tissue Res 303:235–252. doi:10.1007/s004410000296
Ito T, Yamakawa H, Bregonzio C, Terrón JA, Falcón-Neri A, Saavedra JM (2002) Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke 33:2297–2303. doi:10.1161/01.STR.0000027274.03779.F3
Jöhren O, Saavedra JM (1996) Expression of AT1A and AT1B angiotensin II receptor messenger RNA in forebrain of 2-wk-old rats. Am J Physiol 271:E104–E112
Konsman JP, Parnet P, Dantzer R (2002) Cytokine-induced sickness behavior: mechanisms and implications. Trends Neurosci 25:154–159. doi:10.1016/S0166-2236(00)02088-9
Laflamme N, Rivest S (2001) Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J 15:155–163. doi:10.1096/fj.00-0339com
Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T (2003) Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA 100:8514–8519. doi:10.1073/pnas.1432609100
Lemarié CA, Paradis P, Schiffrin EL (2008) New insights on signaling cascades induced by cross-talk between angiotensin II and aldosterone. J Mol Med 86:673–678. doi:10.1007/s00109-008-0323-5
Marchesi C, Paradis P, Schiffrin EL (2008) Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci 29:367–374. doi:10.1016/j.tips.2008.05.003
Mehta PK, Griendling KK (2007) Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292:C82–C97. doi:10.1152/ajpcell.00287.2006
Miyamoto S, Verma IM (1995) Rel/NF-kappa B/I kappa B story. Adv Cancer Res 66:255–292. doi:10.1016/S0065-230X(08)60257-2
Miyazaki A, Kitaichi N, Ohgami K, Iwata D, Jin XH, Iwabuchi K, Morohashi T, Ohno S, Onoe K (2008) Anti-inflammatory effect of angiotensin type 1 receptor antagonist on endotoxin-induced uveitis in rats. Graefes Arch Clin Exp Ophthalmol 246:747–757
Miyoshi M, Nagata K, Imoto T, Goto O, Ishida A, Watanabe T (2003) ANG II is involved in the LPS-induced production of proinflammatory cytokines in dehydrated rats. Am J Physiol Regul Integr Comp Physiol 284:R1092–R1097
Miyoshi M, Miyano K, Moriyama N, Taniguchi M, Watanabe T (2008) Angiotensin type 1 receptor antagonist inhibits lipopolysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor kappa B and activator protein-1 activation. Eur J NeuroSci 27:343–351
Moncek F, Aguilera G, Jezova D (2003) Insufficient activation of adrenocortical but not adrenomedullary hormones during stress in rats subjected to repeated immune challenge. J Neuroimmunol 142:86–92. doi:10.1016/S0165-5728(03)00268-6
Nadeau S, Rivest S (2003) Glucocorticoids play a fundamental role in protecting the brain during innate immune response. J Neurosci 23:5536–5544
Nishimura Y, Ito T, Hoe K, Saavedra JM (2000a) Chronic peripheral administration of the angiotensin II AT(1) receptor antagonist candesartan blocks brain AT(1) receptors. Brain Res 871:29–38. doi:10.1016/S0006-8993(00)02377-5
Nishimura Y, Ito T, Saavedra JM (2000b) Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke 31:2478–2486
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462. doi:10.1002/glia.20467
Quan N (2008) Immune-to-brain signaling: how important are the blood–brain barrier-independent pathways? Mol Neurobiol 37:142–152. doi:10.1007/s12035-008-8026-z
Quan N, Banks WA (2007) Brain-immune communications pathways. Brain Behav Immun 21:727–735. doi:10.1016/j.bbi.2007.05.005
Quan N, Whiteside M, Kim L, Herkenham M (1997) Induction of inhibitory factor kappaBalpha mRNA in the central nervous system after peripheral lipopolysaccharide administration: an in situ hybridization histochemistry study in the rat. Proc Natl Acad Sci USA 94:10985–10990. doi:10.1073/pnas.94.20.10985
Quan N, Stern EL, Whiteside MB, Herkenham M (1999) Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol 93:72–80. doi:10.1016/S0165-5728(98)00193-3
Quan N, He L, Lai W (2003) Endothelial activation is an intermediate step for peripheral lipopolysaccharide induced activation of paraventricular nucleus. Brain Res Bull 59:447–452. doi:10.1016/S0361-9230(02)00951-6
Rivest S (2003) Molecular insights on the cerebral innate immune system. Brain Behav Immun 17:13–19. doi:10.1016/S0889-1591(02)00055-7
Rivest S, Lacroix S, Vallières L, Nadeau S, Zhang J, Laflamme N (2000) How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med 223:22–38. doi:10.1046/j.1525-1373.2000.22304.x
Rogers J, Mastroeni D, Leonard B, Joyce J, Grover A (2007) Neuroinflammation in Alzheimer’s disease and Parkinson’s disease: are microglia pathogenic in either disorder? Int Rev Neurobiol 82:235–246. doi:10.1016/S0074-7742(07)82012-5
Saavedra JM (2005) Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol 25:485–512. doi:10.1007/s10571-005-4011-5
Saavedra JM, Benicky J (2007) Brain and peripheral angiotensin II play a major role in stress. Stress 10:185–193. doi:10.1080/10253890701350735
Saavedra JM, Nishimura Y (1999) Angiotensin and cerebral blood flow. Cell Mol Neurobiol 19:553–573. doi:10.1023/A:1006995016403
Saavedra JM, Armando I, Bregonzio C, Juorio A, Macova M, Pavel J, Sánchez-Lemus E (2006a) A centrally acting, anxiolytic angiotensin II AT1 receptor antagonist prevents the isolation stress-induced decrease in cortical CRF1 receptor and benzodiazepine binding. Neuropsychopharm 31:1123–1134
Saavedra JM, Benicky J, Zhou J (2006b) Angiotensin II: multitasking in the brain. J Hypertens 24(Suppl):S131–S137. doi:10.1097/01.hjh.0000220418.09021.ee
Saavedra JM, Benicky J, Zhou J (2006c) Mechanisms of the anti-ischemic effect of angiotensin II AT (1) receptor antagonists in the brain. Cell Mol Neurobiol 26:1099–1111
Sánchez-Lemus E, Murakami Y, Larrayoz-Roldan IM, Moughamian AJ, Pavel J, Nishioku T, Saavedra JM (2008) Angiotensin II AT1 receptor blockade decreases lipopolysaccharide-induced inflammation in the rat adrenal gland. Endocrinology 149:5177–5188. doi:10.1210/en.2008-0242
Sanvitto GL, Jöhren O, Häuser W, Saavedra JM (1997) Water deprivation upregulates ANG II AT1 binding and mRNA in rat subfornical organ and anterior pituitary. Am J Physiol 273:E156–E163
Sanz-Rosa D, Oubiña MP, Cediel E, de las Heras N, Vegazo O, Jiménez J, Lahera V, Cachofeiro V (2005) Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: role of NF-κB/IκB system. Am J Physiol Heart Circ Physiol 288:H111–H115. doi:10.1152/ajpheart.01061.2003
Savoia C, Schiffrin EL (2006) Inflammation in hypertension. Curr Opin Nephrol Hypertens 15:152–158. doi:10.1097/01.mnh.0000203189.57513.76
Savoia C, Schiffrin EL (2007) Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci 112:375–384. doi:10.1042/CS20060247
Shlyakhto E (2007) Observational Study on Cognitive function And systolic blood pressure Reduction (OSCAR): preliminary analysis of 6-month data from >10, 000 patients and review of the literature. Curr Med Res Opin 23(Suppl 5):S13–S18. doi:10.1185/030079907X260719
Singh AK, Jiang Y (2004) How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology 201:197–207. doi:10.1016/j.tox.2004.04.015
Stier CT Jr, Rocha R, Chander PN (2005) Effect of aldosterone and MR blockade on the brain and the kidney. Heart Fail Rev 10:53–62. doi:10.1007/s10741-005-2349-x
Streit WJ, Walter SA, Pennell NA (1999) Reactive microgliosis. Prog Neurobiol 57:563–581. doi:10.1016/S0301-0082(98)00069-0
Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J (2003) Inflammation and angiotensin II. Int J Biochem Cell Biol 35:881–900. doi:10.1016/S1357-2725(02)00271-6
Triantafilou M, Triantafilou K (2005) The dynamics of LPS recognition: complex orchestration of multiple receptors. J Endotoxin Res 11:5–11
Tsutsumi K, Saavedra JM (1991a) Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Physiol 261:R209–R216
Tsutsumi K, Saavedra JM (1991b) Angiotensin-II receptor subtypes in median eminence and basal forebrain areas involved in regulation of pituitary function. Endocrinology 129:3001–3008
Tsutsumi K, Strömberg C, Saavedra JM (1992) Characterization of angiotensin II receptor subtypes in the rat spleen. Peptides 13:291–296. doi:10.1016/0196-9781(92)90111-F
Vakharia K, Hinson JP (2005) Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology 146:1398–1402. doi:10.1210/en.2004-0882
Wluka A, Olszewski WL (2006) Innate and adaptive processes in the spleen. Ann Transplant 11:22–29
Xia Y, Yamagata K, Krukoff TL (2006) Differential expression of the CD14/TLR4 complex and inflammatory signaling molecules following i.c.v. administration of LPS. Brain Res 109:85–95. doi:10.1016/j.brainres.2006.03.112
Yamakawa H, Jezova M, Ando H, Saavedra JM (2003) Normalization of endothelial and inducible nitric oxide synthase expression in brain microvessels of spontaneously hypertensive rats by angiotensin II AT1 receptor inhibition. J Cereb Blood Flow Metab 23:371–380. doi:10.1097/00004647-200303000-00012
Zhou J, Ando H, Macova M, Dou J, Saavedra JM (2005) Angiotensin II AT1 receptor blockade abolishes brain microvascular inflammation and heat shock protein responses in hypertensive rats. J Cereb Blood Flow Metab 25:878–886. doi:10.1038/sj.jcbfm.9600082
Zhou J, Pavel J, Macova M, Yu ZX, Imboden H, Ge L, Nishioku T, Dou J, Delgiacco E, Saavedra JM (2006) AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke 37:1271–1276. doi:10.1161/01.STR.0000217404.64352.d7
Acknowledgments
This research was supported by the Division of Intramural Research Programs, National Institute of Mental Health, National Institutes of Health, Department of Health and Human Services, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benicky, J., Sánchez-Lemus, E., Pavel, J. et al. Anti-Inflammatory Effects of Angiotensin Receptor Blockers in the Brain and the Periphery. Cell Mol Neurobiol 29, 781–792 (2009). https://doi.org/10.1007/s10571-009-9368-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9368-4