Abstract
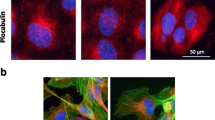
TR-644 is a novel combretastatin A-4 (CA-4) analogue endowed with potent microtubule depolymerizing activity superior to that of the lead compound and it also has high affinity to colchicines binding site of tubulin. We tested TR-644 anti-angiogenic effects in human umbilical endothelial cells (HUVEC). It showed no significant effects on the growth of HUVEC cells at concentrations below 1,000 nM, but at much lower concentrations (10–100 nM) it induced inhibition of capillary tube formation, inhibition of endothelial cell migration and affected endothelial cell morphology as demonstrated by the disruption of the microtubule network. TR-644 also increased permeability of HUVEC cells in a time dependent manner. The molecular mechanism for the anti-vascular activity of TR-644 was investigated in detail. TR-644 caused G2/M arrest in endothelial cells and this effect correlated with downregulation of the expression of Cdc25C and Cdc2Tyr15. Moreover TR-644 inhibited VEGF-induced phosphorylation of VE-cadherin but did not prevent the VEGF-induced phosphorylation of FAK. In chick chorioallantoic membrane in vivo assay, TR-644 (0.1–1.0 pmol/egg) efficiently counteracted the strong angiogenic response induced by FGF. Also CA-4, used as reference compound, caused an antagonistic effect, but in contrast, it induced per se, a remarkable angiogenic response probably due to an inflammatory reaction in the site of treatment. In a mice allogenic tumor model, immunohistochemical staining of tumors with anti-CD31 antibody showed that TR-644 significantly reduced the number of vessel, after 24 h from the administration of a single dose (30 mg/Kg).











Similar content being viewed by others
References
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Dumontet C, Jordan MA (2010) Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 9:790–803
Tozer GM, Kanthou C, Baguley BC (2005) Disrupting tumor blood vessel. Nat Rev Cancer 5:423–435
Schwartz EL (2009) Antivascular actions of microtubule-binding drugs. Clin Cancer Res 15:2594–2601
Grosios K, Holwell SE, McGown AT, Pettit GR, Bibby MC (1999) In vivo and in vitro evaluation of combretastatin A-4 and its sodium phosphate prodrug. Br J Cancer 81:1318–1327
Gaukroger K, Hadfield JA, Lawrence NJ, Nlan S, McGown AT (2003) Structural requirements for the interaction of combretastatins with tubulin: how important is the trimethoxy unit? Org Biomol Chem 1:3033–3037
Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA (2006) Medicinal chemistry of combretastatin A4: present and future directions. J Med Chem 49:3033–3044
Wang L, Woods KW, Li Q, Barr KJ, McCroskey RW, Hannick SM, Gherke L, Credo RB, Hui YH, Marsh K, Warner R, Lee JY, Zielinski-Mozng N, Frost D, Rosenberg SH, Sham HL (2002) Potent, orally active heterocycle-based combretastatin A-4 analogues: synthesis, structure-activity relationship, pharmacokinetics, and in vivo antitumor activity evaluation. J Med Chem 45:1697–1711
Schobert R, Biersack B, Dietrich A, Effenberger K, Knauer S, Mueller T (2010) 4-(3-Halo/amino-4,5-dimethoxyphenyl)-5-aryloxazoles and N-methylimidazoles that are cytotoxic against combretastatin A resistant tumor cells and vascular disrupting in a cis platin resistant germ cell tumor model. J Med Chem 53:6595–6602
Ohsumi K, Hatanaka T, Fujita K, Nakagawa R, Fukuda Y, Nihai Y, Suga Y, Morinaga Y, Akiyama Y, Tsuji T (1988) Synthesis and antitumor activity of cis-restricted combretastatins 5-membered heterocyclic analogues. Bioorg Med Chem Lett 8:3153–3158
Tron GC, Pagliai F, Del Grosso E, Genazzani AA, Sorba G (2005) Synthesis and cytotoxic evaluation of combretafurazans. J Med Chem 48:3260–3268
Liu T, Dong X, Xue N, Wu R, He Q, Yang B, Hu Y (2009) Synthesis and biological evaluation of 3,4-biaryl-5-aminoisoxazole derivatives. Bioorg Med Chem 17:6279–6285
Wu M, Li W, Yang C, Chen D, Ding J, Chen Y, Lin L, Xie Y (2007) Synthesis and activity of combretastatin A-4 analogues: 1,2,3-thiadiazoles as potent antitumor agents. Bioorg Med Chem Lett 17:869–873
Romagnoli R, Baraldi PG, Cruz-Lopez O, Lopez-Cara C, Carrion MD, Brancale A, Hamel E, Chen L, Bortolozzi R, Basso G, Viola G (2010) Synthesis and antitumor activity of 1,5-disubstituted 1,2,4-triazoles as cis-restricted combretastatin analogs. J Med Chem 53:4248–4258
Romagnoli R, Baraldi PG, Brancale A, Ricci A, Hamel E, Bortolozzi R, Basso G, Viola G (2011) Convergent synthesis and biological evaluation of 2-amino-4-(3′,4′,5′-trimethoxyphenyl)-5-aryl thiazoles as microtubule targeting agents. J Med Chem 54:5144–5153
Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D (1989) Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experentia 45:209–211
Pettit GR, Temple C Jr, Narayanan VL, Varma R, Boyd MR, Rener GA, Bansal N (1995) Antineoplastic agents 322. Synthesis of combretastatin A-4 prodrugs. Anticancer Drug Des 10:299–309
Pettit GR, Singh SB, Boyd MR, Hamel E, Pettit RK, Schmidt JM, Hogan F (1985) Antineoplastic agents. 291. Isolation and synthesis of combretastatins A-4, A-5, and A-6(1a). J Med Chem 38:1666–1672
Mitola S, Strasly M, Prato M, Ghia P, Bussolino F (2003) IL-12 regulates an endothelial cell-lymphocyte network: effect on metalloproteinase-9 production. J Immunol 171:3725–3733
Basili S, Basso G, Faccio A, Granzhan A, Ihmels H, Moro S, Viola G (2008) Diazoniapolycyclic ions inhibit activity of topoisomerase I and growth of certain tumor cell lines. ChemMedChem 3:1671–1676
Liang CC, Park AY, Guan JL (2007) In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2:329–333
Guidolin D, Vacca A, Nussdorfer GG, Ribatti D (2004) A new image analysis method based on topological and fractal parameters to evaluate the angiostatic activity of docetaxel by using the Matrigel assay in vitro. Microvasc Res 67:117–124
Viola G, Fortunato E, Cecconet L, Del Giudice L, Dall’Acqua F, Basso G (2008) Central role of mitochondria and p53 in PUVA-induced apoptosis in human keratinocytes cell line NCTC-2544. Toxicol Appl Pharmacol 227:84–96
Chiodelli P, Mitola S, Ravelli C, Oreste P, Rusnati M, Presta M (2011) Heparan sulfate proteoglycans mediate the angiogenic activity of the vascular endothelial growth factor receptor-2 agonist gremlin. Arterioscler Thromb Vasc Biol 31:e116–e127
Dejana E, Giampietro C (2012) Vascular endothelial-cadherin and vascular stability. Curr Opin Hematol 19:218–223
Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P (1997) Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci USA 94:6273–6278
Feraud O, Cao Y, Vittet D (2001) Embryonic stem cell-derived embryoid bodies development in collagen gels recapitulates sprouting angiogenesis. Lab Invest 81:1669–1681
Dejana E, Orsenigo F, Lampugnani MG (2008) The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 121:2115–2122
Vincent L, Kermani P, Young LM, Cheng J, Zhang F, Shido K, Lam G, Bompais-Vincent H, Zhu Z, Hicklin DJ, Bohlen P, Chaplin DJ, May C, Rafii S (2005) Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling. J Clin Invest 115:2992–3006
Bogatcheva NV, Verin AD (2008) The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res 76:202–207
Mitra SK, Hanson DA, Schlaepfer DD (2005) Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 6:56–68
Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC (2011) VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell 22:2766–2776
Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD (2012) VEGF-induced vascular permeability is mediated by FAK. Dev Cell 22:146–157
Andrés G, Leali D, Mitola S, Coltrini D, Camozzi M, Corsini M, Belleri M, Hirsch E, Schwendener RA, Christofori G, Alcamí A, Presta M (2009) A pro-inflammatory signature mediates FGF2-induced angiogenesis. J Cell Mol Med 13:2083–2108
Atherton-Fessler S, Liu F, Gabrielli B, Lee MS, Peng CY, Piwnica-Worms H (1994) Cell cycle regulation of the p34cdc2 inhibitory kinases. Mol Biol Cell 5:989–1001
Choi HJ, Fukui M, Zhu BT (2012) Role of cyclin B1/Cdc2 up-regulation in the development of mitotic prometaphase arrest in human breast cancer cells treated with nocodazole. PLoS One 6:e24312
Mollinedo F, Gajate C (2003) Microtubules, microtubule-interfering agents and apoptosis. Apoptosis 8:413–450
Lu H, Murtagh J, Schwartz EL (2006) The microtubule binding drug laulimalide inhibits vascular endothelial growth factor-induced human endothelial cell migration and is synergistic when combined with docetaxel (taxotere). Mol Pharmacol 69:1207–1215
Murtagh J, Lu H, Schwartz EL (2006) Taxotere-induced inhibition of human endothelial cell migration is a result of heat shock protein 90 degradation. Cancer Res 66:8192–8199
Qin L, Zhang M (2010) Maspin regulates endothelial cell adhesion and migration through an integrin signaling pathway. J Biol Chem 285:32360–32369
Dalyot-Herman N, Delgado-Lopez F, Gewirtz DA, Gupton JT, Schwartz EL (2009) Interference with endothelial cell function by JG-03-14, an agent that binds to the colchicine site on microtubules. Biochem Pharmacol 78:1167–1177
Katz M, Amit I, Citri A, Shay T, Carvalho S, Lavi S, Milanezi F, Lyass L, Amariglio N, Jacob-Hirsch J, Ben-Chetrit N, Tarcic G, Lindzen M, Avraham R, Liao YC, Trusk P, Lyass A, Rechavi G, Spector NL, Lo SH, Schmitt F, Bacus SS, Yarden Y (2007) A reciprocal tensin-3-cten switch mediates EGF-driven mammary cell migration. Nat Cell Biol 9:961–969
Presta M, Andrés G, Leali D, Dell’Era P, Ronca R (2009) Inflammatory cells and chemokines sustain FGF2-induced angiogenesis. Eur Cytokine Netw 20:39–50
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Elena Porcù and Giampietro Viola: equal contribution.
Rights and permissions
About this article
Cite this article
Porcù, E., Viola, G., Bortolozzi, R. et al. TR-644 a novel potent tubulin binding agent induces impairment of endothelial cells function and inhibits angiogenesis. Angiogenesis 16, 647–662 (2013). https://doi.org/10.1007/s10456-013-9343-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-013-9343-z