Summary
Objective
Connection between abdominal obesity, type 2 diabetes, and the hypothalamic-pituitary-adrenal (HPA) axis activity remains unclear. The aim of this study was to measure HPA axis activity in 121 type 2 diabetics, in 29 obese subjects, and 19 control subjects.
Research design and methods
Physical examination, anthropometric measures, psychological questionnaire, psychiatric interview, neurological and ophthalmologic examination were performed. Biochemical parameters, urinary free cortisol levels (UFC), cortisol and ACTH levels at 8 and 16 h, cortisol levels after overnight suppression with 1 mg dexamethasone followed by ACTH test in 30 and 60 min were measured. Groups were stratified in relation to obesity, body fat distribution, and chronic complications.
Results
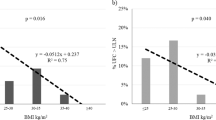
UFC and postdexamethasone cortisol were significantly increased in diabetic patients compared with both obese subjects (p < 0.01) and control group (p < 0.05), regardless to diabetic complications and obesity. Postdexamethasone cortisol was correlated with waist circumference. ACTH-induced cortisol levels were significantly higher in all type 2 diabetic patients. An independent association was found between AUC cortisol in ACTH test and insulin resistance. Multiple regression analysis showed that waist circumference was independently associated with sex, fasting plasma insulin, morning cortisol, and AUC of cortisol in ACTH test (R 2 = 0.334,p < 0.0000).
Conclusions
In type 2 diabetic patients, the HPA axis is clearly hyperactive as evident in increased urinary free cortisol, diminished cortisol suppression after dexamethasone and increased ACTH-induced cortisol levels. Abdominal obesity and the presence of chronic complications increased the HPA axis hyperactivity in type 2 diabetes. Augmentation of positive feedback is associated with insulin resistance and negative feedback with abdominal obesity.
Zusammenfassung
Ziel
Es besteht Unklarheit über die Beziehung zwischen abdominaler Adipositas, Diabetes mellitus Typ 2 und der Hypothalamus-Hypophysen-Nebennieren (HHNN) Achse. Ziel dieser Studie war es, die Aktivität der HHNN-Achse bei 121 Typ 2 Diabetikern, 29 adipösen und 19 Kontrollpersonen zu messen.
Studiendesign und Methodik
Es wurden eine physikalische Untersuchung, anthropometrische Messungen, psychologische Befragungen, psychiatrische Interviews sowie neurologische und ophthalmologische Untersuchungen durchgeführt. Außerdem wurden biochemische Parameter, freie Cortisol Spiegel im Urin (FCU), Cortisol und ACTH Konzentrationen um 8 und um 16 Uhr, sowie Cortisol Konzentrationen nach spätabendlicher Gabe von 1 mg Dexamethason zur Suppression, gefolgt von einem ACTH Test mit Cortisol Messung 30 und 60 min nach der Gabe erhoben. Die Gruppeneinteilung erfolgte in Bezug auf Adipositas, Körper -Fettverteilung und dem Vorhandensein chronischer Komplikationen.
Ergebnisse
Unabhängig von diabetischen Komplikationen und Adipositas waren die FCU und das Post-Dexamethason Cortisol bei den Diabetikern im Vergleich zu den adipösen Patienten (p < 0,01) und der Kontrollgruppe (p < 0,05) erhöht. Die Post-Dexamethason Cortisol Werte waren mit dem Hüftumfang korreliert. Die ACTH stimulierten Cortisol Werte waren bei allen Typ 2 Diabetikern significant höher. Es wurde eine unabhängige Beziehung zwischen der AUC der Cortisolreaktion im ACTH Test und der Insulinresistenz gefunden. Die multiple Regressionsanalyse zeigte, dass der Hüftumfang unabhängig mit dem Geschlecht, dem Nüchtern-Insulin, dem morgendlichem Cortisol und der AUC des Cortisols im ACTH Test assoziiert war (R 2 = 0.334,p < 0.0000).
Schlussfolgerungen
Die erhöhten FCU Werte, die verminderte Cortisol Suppression durch Dexamethason sowie die gesteigerte Reaktion der Cortisolspiegel auf ACTH Gabe belegen klar eine Hyperaktivität der HHNN-Achse bei Typ 2 Diabetikern. Eine abdominale Adipositas und chronische Komplikationen steigerten die Hyperaktivität der HHNN-Achse bei Typ 2 Diabetikern. Eine Steigerung des positiven Feedbacks ist mit Insulinresistenz und des negativen Feedbacks mit abdominaler Adipositas assoziiert.
Similar content being viewed by others
References
DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94.
Boscaro M, Barzon I, Fallo F, Sonino N. Cushing’s syndrome. Lancet. 2001;357:783–91.
Connell JMC, Whitworth JA, Davies DL, Lever AF, Richards AM, Fraser R. Effects of ACTH and cortisol administrations on blood pressure, electrolyte metabolism, atrial natriuretic peptide and renal function in normal man. J Hypertens. 1986;5:425–33.
Bjorntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and type 2 diabetes mellitus. Diabetic Med. 1999;16:373–81.
Hüther KJ, Scholz HR. The plasma concentrations and rate of plasma clearance and plasma production of cortisol and corticosterone in healthy persons and in subjects with asymptomatic and clinical diabetes mellitus. Horm Metab Res. 1969;1:253.
Cameron OG, Kronfol Z, Greden JF, Carroll BJ. Hypothalamic-pituitary-adrenocortical activity in patients with diabetes mellitus. Arch Gen Psychiatry. 1984;41:1090–5.
Hudson JI, Hudson MS, Rotschils AJ, Vignati L, Schatzberg AF, Melby JC. Abnormal results of dexamethasone suppression tests in nondepressed patients with diabetes mellitus. Arch Gen Psychiatry. 1984;41:1086–9.
Tsigos C, Young RJ, White A. Diabetic neuropathy is associated with increased activity of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 1993;76:554–8.
Liu H, Bravata DM, Cabaccan J, Raffa H, Ryzen E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin Endocrinol (Oxf). 2005;63:642–9.
Roy MS, Roy A, Brown S. Increased urinary free cortisol outputs in diabetic patients. J Diabetes Complicat. 1998;12:24–7.
Dacou-Voutetakis C, Peppa-Patrikiou M, Dracopoulou M. Urinary free cortisol and its nyctohemeral variations in adolescent and young adults with IDDM: relation to endothelin 1 and indices of diabetic angiopathy. J Pediatr Endocrinol. 1998;11:437–45.
Oltmanns KM, Dodt B, Raspe HH, Schweiger U, Born J, Fehm H, et al. Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. Eur J Endocrinol. 2006;154:325–31.
Lee ZSK, Chan JCN, Yeung VTF, Chow CC, Lau MSW, Ko GTC, et al. Plasma insulin, growth hormone, cortisol, and central obesity among young Chinese type 2 diabetic patients. Diabetes Care. 1999;22:1450–7.
Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, et al. Cortisol secretion in patients with type 2 diabetes: relationship with chronic complications. Diabetes Care. 2007;30:83–8.
Putigano P, Duini A, Toja P, Inviti C, Bonfanti S, Raedelli G, et al. Salivatory cortisol measurement in normal-weight, obese and anorectic women: comparison with plasma cortisol. Eur J Endocrinol. 2001;145:165–71.
Haffner SM, Gonzales C, Mietinen H, Kennedy E, Stern MP. A prospective analysis of the HOMA model. The Mexico city diabetes study. Diabetes Care. 1996;19:1138–41.
Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J. 1985;285:916–8.
Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Green DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–9.
Chiodera P. Low-dose ovine corticotropin-releasing hormone stimulation test in diabetes mellitus with or without neuropathy. Metabolism. 1995;44:538–42.
Akama SF, Cascio CS, Du JZ, Levin N, Dallman MF. Reset of feedback in the adrenocortical system: an apparent shift in sensitivity of adrenocorticotropin to inhibition by corticosterone between morning and evening. Endocrinology. 1986;119:2325–32.
Pavlovcik RA, Phillips MI, Kappy MS, Raiyada MK. Insulin inhibits pyramidal neurons in hypocampal slices. Brain Res. 1984;309:187–91.
Jacobsen L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–34.
Fruehwald-Schuletes B, Kern W, Bong W, Wellhoener P, Kerner W, Born J, et al. Supraphysiological hyperinsulinemia acutely increases hypothalamic-pituitary-adrenal secretory activity in humans. J Clin Endocrinol Metab. 1999;84:3041–6.
Chan O, Inouye K, Vranic M, Matthew SG. Hyperactivation of the hypothalamo-pituitary-adrenocortical axis in streptozotocin-diabetes is associated with reduced stress responsiveness and decreased pituitary and adrenal sensitivity. Endocrinology. 2002;143:1761–8.
Chan O, Chan S, Inouye K, Shum K, Matthew SG, Vranic M. Diabetes impairs hypothalamo-pituitary-adrenal (HPA) response to hypoglycemia, and insulin treatment normalizes HPA but not epinephrine response. Diabetes. 2002;51:1681–9.
Kaye TB, Rubin RA, Goldfine AB, Rajamani K, Kinsley BT, Vischer UM, et al. Effect of glycemic control on the overnight dexamethasone suppression test in patients with diabetes mellitus. J Clin Endocrinol Metab. 1992;74:640–4.
Fraser R, Ingram MC, Anderson NH, Morrison C, Davies E, Connell JMC. Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension. 1999;33:1364–8.
Reynolds RM, Labad J, Strachan MW, Braun A, Fowkes FG, Lee AJ, et al. Elevated fasting plasma cortisol is associated with ischemic heart disease and its risk factors in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. J Clin Endocrinol Metab. 2010;95:1602–8.
Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26(Suppl 1):31–5.
Bruehl H, Rueger M, Dziobek I, Sweat V, Tirsi A, Javier E, et al. Hypothalamic-pituitary-adrenal axis dysregulation and memory impairments in type 2 diabetes. J Clin Endocrinol Metab. 2007;92:2439–45.
Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009;1280:186–94.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prpić-Križevac, I., Canecki-Varžić, S. & Bilić-Ćurčić, I. Hyperactivity of the hypothalamic-pituitary-adrenal axis in patients with type 2 diabetes and relations with insulin resistance and chronic complications. Wien Klin Wochenschr 124, 403–411 (2012). https://doi.org/10.1007/s00508-012-0191-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00508-012-0191-4
Schlüsselwörter
- Cortisol Sekretion
- Diabetes mellitus Typ 2
- Insulinresistenz
- Abdominale Adipostas
- chronische Komplikationen