Abstract
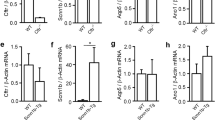
Cystic fibrosis (CF) is caused by genetic mutations that lead to dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel. The most common mutation, ΔF508, causes inefficient trafficking of mutant CFTR protein from the endoplasmic reticulum to the cell membrane. Therapeutic efforts have been aimed at increasing the level of ΔF508-CFTR protein in the membrane using agents such as sodium butyrate. In this study, we investigated the effects of culturing a human airway epithelial cell line, Calu-3, in the presence of 5 mM sodium butyrate. Within 24 h, butyrate exposure caused a significant decrease in the basal, as well as Ca2+-activated, anion secretion by Calu-3 cell monolayers, determined by the change in transepithelial short-circuit current in response to the Ca2+-elevating agent thapsigargin. The secretory response to 1-ethyl-2-benzimidazolinone, an activator of the basolateral Ca2+-activated K+ channel KCNN4, was similarly reduced by butyrate treatment. Quantitative PCR revealed that these functional effects were associated with dramatic decreases in mRNA for both KCNN4 and CFTR. Furthermore, the KCNQ1 K+ channel was upregulated after butyrate treatment. We suggest that prolonged exposure to sodium butyrate downregulates the expression of both KCNN4 and CFTR, leading to a functional loss of Ca2+-activated anion secretion. Thus, butyrate may inhibit, rather than stimulate, the anion secretory capacity of human epithelial cells that express wild-type CFTR, particularly in tissues that normally exhibit robust Ca2+-activated secretion.





Similar content being viewed by others
References
Berger J, Moller DE (2002) The mechanism of action of PPARs. Annu Rev Med 53:409–435
Bernard K, Bogliolo S, Soriani J, Ehrenfeld J (2003) Modulation of calcium-dependent chloride secretion by basolateral SK-4-like channels in a human bronchial cell line. J Membr Biol 196:15–31
Bleich M, Warth R (2000) The very small-conductance K+ channel KvLQT1 and epithelial function. Pflügers Arch 440:202–206
Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE (1990) Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63:827–834
Cheng SH, Fang SL, Zabner J, Marshall J, Piraino S, Schiavi SC, Jefferson DM, Welsh MJ, Smith AE (1995) Functional activation of the cystic fibrosis trafficking mutant ΔF508-CFTR by overexpression. Am J Physiol 268:L615–L624
Cotton CU (2000) Basolateral potassium channels and epithelial ion transport. Am J Respir Cell Mol Biol 23:270–272
Cowley EA (2003) Isoprostane-mediated secretion from human airway epithelial cells. Mol Pharmacol 64:298–307
Cowley EA, Linsdell P (2002) Characterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J Physiol 538:747–757
Cowley EA, Linsdell P (2002) Oxidant stress stimulates anion secretion from the human airway epithelial cell line Calu-3: implications for cystic fibrosis lung disease. J Physiol 543:201–209
Davie JR (2003) Inhibition of histone deacetylase activity by butyrate. J Nutr 133:2485S–2493S
Devor DC, Singh AK, Lambert LC, Deluca A, Frizzell RA, Bridges RA (1999) Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol 133:743–760
Heda GD, Tanwani M, Marino CR (2001) The ΔF508 mutation shortens the biochemical half-life of plasma membrane CFTR in polarized epithelial cells. Am J Physiol 280:C166–C174
Hihi AK, Michalik L, Wahli W (2002) PPARs: transcriptional effectors of fatty acids and their derivatives. Cell Mol Life Sci 59:790–798
Jensen BS, Strobaek D, Olesen S-P, Christophersen P (2001) The Ca2+-activated K+ channel of intermediate conductance: a molecular target for novel treatments? Current Drug Targets 2:401–422
Joiner WJ, Basavappa S, Vidyasagar S, Nehrke K, Krishnan S, Binder HJ, Boulpaep EL, Rajendran VM (2003) Active K+ secretion through multiple Kca-type channels and regulation by IKCa channels in rat proximal colon. Am J Physiol 285:G185–G196
Kopito RR (1999) Biosynthesis and degradation of CFTR. Physiol Rev 79:S167–S173
Kunzelmann K, Mall M (2001) Pharmacotherapy of the ion transport defect in cystic fibrosis. Clin Exp Pharmacol Physiol 28:857–867
Li C, Ramjeesingh M, Reyes E, Jensen T, Chang X, Rommens JM, Bear CE (1993) The cystic fibrosis mutation (ΔF508) does not influence the chloride channel activity of CFTR. Nat Genet 3:311–316
Linsdell P (2001) Direct block of the cystic fibrosis transmembrane conductance regulator Cl− channel by butyrate and phenylbutyrate. Eur J Pharmacol 411:255–260
Loffing J, Moyer BD, Reynolds D, Stanton BA (1999) PBA increases CFTR expression but at high doses inhibits Cl− secretion in Calu-3 airway epithelial cells. Am J Physiol 277:L700–L708
MacVinish LJ, Keogh J, Cuthbert AW (2001) EBIO, an agent causing maintained epithelial chloride secretion by co-ordinate actions at both apical and basolateral membranes. Pflügers Arch 443:S127–S131
Mall M, Kunzelmann K (2005) Correction of the CF defect by curcumin: hypes and disappointments. Bioessays 27:9–13
Mariadson JM, Corner GA, Augenlicht LH (2000) Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res 60:4561–4572
Moon S, Singh S, Krouse ME, Wine JJ (1997) Calcium-stimulated Cl− secretion in Calu-3 human airway cells requires CFTR. Am J Physiol 273:L1208–L1219
Moyer BD, Loffing-Cueni D, Loffing J, Reynolds D, Stanton BA (1999) Butyrate increases apical membrane CFTR but reduces chloride secretion in MDCK cells. Am J Physiol 277:F271–F276
Pilewski JM, Frizzell RA (1999) Role of CFTR in airway disease. Physiol Rev 79:S215–S255
Powell K, Zeitlin PL (2002) Therapeutic approaches to repair defects in ΔF508 CFTR folding and cellular targeting. Adv Drug Deliv Rev 54:1395–1408
Rubenstein RC, Egan ME, Zeitlin PL (1997) In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing ΔF508-CFTR. J Clin Invest 100:2457–2465
Rubenstein RC, Zeitlin PL (1998) A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in ΔF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med 157:484–490
Shen B-Q, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH (1994) Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am J Physiol 266:L493–L501
Sheppard DN, Welsh MJ (1999) Structure and function of the CFTR chloride channel. Physiol Rev 79:S23–S45
Wang ACC, Dai X, Luu B, Conrad DJ (2001) Peroxisome proliferator-activated receptor-γ regulates airway epithelial cell activation. Am J Respir Cell Mol Biol 24:688–693
Zeitlin PL (2000) Pharmacological restoration of ΔF508 CFTR-mediated chloride current. Kidney Int 57:832–837
Zeitlin PL, Diener-West M, Rubenstein RB, Boyle MP, Lee CK, Brass-Ernst L (2002) Evidence of CFTR function in cystic fibrosis after systemic administration of 4-phenylbutyrate. Mol Ther 6:119–126
Acknowledgements
This work was supported by grants from the Canadian Cystic Fibrosis Foundation (to P.L.) and the Natural Science and Engineering Research Council (to E.A.C.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roy, J., Denovan-Wright, E.M., Linsdell, P. et al. Exposure to sodium butyrate leads to functional downregulation of calcium-activated potassium channels in human airway epithelial cells. Pflugers Arch - Eur J Physiol 453, 167–176 (2006). https://doi.org/10.1007/s00424-006-0128-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-006-0128-8