Abstract
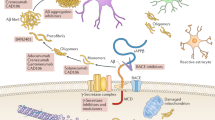
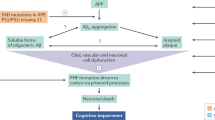
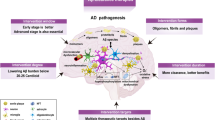
The amyloid cascade hypothesis is widely accepted as the centerpiece of Alzheimer disease (AD) pathogenesis. It proposes that abnormal production of beta amyloid protein (Abeta) is the cause of AD and that the neurotoxicity is due to Abeta itself or its oligomeric forms. We suggest that this, in itself, cannot be the cause of AD because demonstrating such toxicity requires micromolar concentrations of these Abeta forms, while their levels in brain are a million times lower in the picomolar range. AD probably results from the inflammatory response induced by extracellular Abeta deposits, which later become enhanced by aggregates of tau. The inflammatory response, which is driven by activated microglia, increases over time as the disease progresses. Disease-modifying therapeutic attempts to date have failed and may continue to do so as long as the central role of inflammation is not taken into account. Multiple epidemiological and animal model studies show that NSAIDs, the most widely used antiinflammatory agents, have a substantial sparing effect on AD. These studies provide a proof of concept regarding the anti-inflammatory approach to disease modification. Biomarker studies have indicated that early intervention may be necessary. They have established that disease onset occurs more than a decade before it becomes clinically evident. By combining biomarker and pathological data, it is possible to define six phases of disease development, each separated by about 5 years. Phase one can be identified by decreases in Abeta in the CSF, phase 2 by increases of tau in the CSF plus clear evidence of Abeta brain deposits by PET scanning, phase 3 by slight decreases in brain metabolic rate by PET-FDG scanning, phase 4 by slight decreases in brain volume by MRI scanning plus minimal cognitive impairment, phase 5 by increased scanning abnormalities plus clinical diagnosis of AD, and phase 6 by advanced AD requiring institutional care. Utilization of antiinflammatory agents early in the disease process remains an overlooked therapeutic opportunity. Such agents, while not preventative, have the advantage of being able to inhibit the consequences of both Abeta and tau aggregation. Since there is more than a decade between disease onset and cognitive decline, a window of opportunity exists to introduce truly effective disease-modifying regimens. Taking advantage of this opportunity is the challenge for the future.


Similar content being viewed by others
References
ADAPT Research Group (2006) Cardiovascular and cerebrovascular events in the randomized, controlled Alzheimer’s disease anti-inflammatory prevention trial (ADAPT). PLOS. doi:10.1371/journal.pctr.0010033
Aisen PS, Gauthier S, Ferris SH et al (2011) Tramiprosate in mild to moderate Alzheimer’s disease- a randomized, double blind, placebo-controlled, multi-center study (the Alphase study). Arch Med Sci 7:102–104
Aisen PS, Schafer KA, Grundman M et al (2003) Effects of rofecoxib or naproxen vs. placebo on Alzheimer’s disease progression: a randomized controlled trial. JAMA 289:2819–2826
Aisen PS, Schmeidler J, Pasinetti GM (2002) Randomized pilot study of nimesulide treatment in Alzheimer’s disease. Neurology 58:1050–1054
Akiyama H, McGeer PL (2004) Specificity of mechanisms for plaque removal after Aβ immunotherapy for Alzheimer disease. Nature Med 10:117–118
Akiyama H, Schwab C, Kondo H et al (1996) Granules in glial cells of patients with Alzheimer’s disease are immunopositive for C-terminal sequences of beta-amyloid protein. Neurosci Lett 206:169–172
Anon (1994) The Canadian Study of Health and Aging: risk factors for Alzheimer’s disease in Canada. Neurology 44: 2073–2080
Anthony JC, Breitner JC, Zandi PP et al (2000) Reduced prevalence of AD in users of NSAIDs and H2 receptor antagonists: the Cache County study. Neurology 54:2066–2071
Arendish GW, Mori T, Cao C et al (2009) Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer’s disease mice. J Alzheimer’s Dis 17:661–680
Arvenitakis Z, Grodstein F, Schneider JA et al (2008) Relation of NSAIDs to incident AD, change in cognitive function and AD pathology. Neurology 70:2219–2225
Ballon C, Griffith LE, Strifler L et al (2012) Vitamin D, cognition, and dementia: a systemic review and meta-analysis. Neurology 79:1397–1405
Bateman RJ, Xiong C, Benziger TLS et al (2012) Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. New Engl J Med 367(9):793–804
Beard CM, Waring SC, O’Brien PC et al (1988) Nonsteroidal anti-inflammatory drug use and Alzheimer’s disease: a case control study in Rochester, Minnesota, 1980 through 1984. Mayo Clin Proc 73:951–955
Bermajo-Parejo F, Antequera D, Vargas JA et al (2010) Saliva levels of Abeta1–42 as potential biomarker of Alzheimer’s disease: a pilot study. BMC Neurol 10:108
Blenow K, Zetterberg H, Rinne JO et al (2012) Effect of immunotherapy with bapineuzumab on cerebral biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol 69:1002–1010
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Zetterberg H, Del Tredici K, Blennow K (2013) Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-b changes occur before increases of tau in spinal fluid. Acta Neuropathol PMID:23756600
Breitner JC, Gau BA, Welsh KA et al (1994) Inverse association of anti-inflammatory treatments and Alzheimer’s disease: initial results of a co-twin control study. Neurology 44:227–232
Breitner JC, Welsh KA, Helms MJ et al (1995) Delayed onset of Alzheimer’s disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. Neurobiol Aging 16:523–530
Brody DL, Magnoni S, Schwetye KE et al (2008) Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 321:1221–1224
Broe GA, Grayson DA, Creasy HM et al (2000) Anti-inflammatory drugs protect against Alzheimer disease at low doses. Arch Neurol 57:1586–1591
Bruck A, Virta JR, Koiyunen J et al (2013) [11C]PIB, {18F}FDG and MY imaging in patients with mild cognitive impairment. Eur J Nucl Med Imaging PMID:23801168
Buchhave P, Minthon L, Zetterberg H et al (2012) Cerebrospinal fluid levels of β-amyloid 1-42, but not of tau, are fully changed already 5 to 10 years before the onset of Alzheimer dementia. Arch Gen Psychiatry 69(1):98–106
Bussiere T, Friend N, Sadegh B et al (2002) Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer’s disease. Neuroscience 112:75–81
Butterfield DA, Griffin S, Munch G, Pasinetti GM (2002) Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer disease brain exists. J Alzheimers Dis 4(3):193–201
Cacquevel M, Aesschbach l, Houacine J, Fraering PC (2012) Alzheimer disease-linked mutations in presenilin-1 result in a drastic loss of activity in purified γ-secretase. PLOS One. doi:10.1371/journal.pone.0035133
Choi SH, Aid S, Caracciolo L et al (2013) Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer’s disease. J Neurochem 124:59–68
Chu YE, Chang WH, Black RM et al (2012) Crude caffeine reduces memory impairment and amyloid β(1-42) levels in an Alzheimer’s mouse model. Food Chem 135:2095–2102
Coric V, van Dyck CH, Salloway S et al (2012) Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol 69:1430–1440
Crehan H, Holton P, Wray S et al (2012) Complement receptor 1 (CR1) and Alzheimer’s disease. Immunobiology 217:244–250
Cribbs DH (2010) Abeta DNA vaccination for Alzheimer’s disease: focus on disease prevention. CNS Neurol Disord Drug Targets 9:207–216
Das P, Murphy MP, Younkin LH et al (2001) Reduced effectiveness of Aβ1-42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiol Aging 22:721–727
DeMattos RB, Bales KR, Cummins DJ et al (2001) Peripheral anti-Abeta antibody alters CNS and plasma Abeta clearance and decreases brain Abeta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 98:8550–8555
Deshpande A, Mina E, Glabe C, Busciglio J (2006) Different conformations of amyloid beta induce neurotoxicity by distinct mechanisms in human cortical neurons. J Neurosci 26:6011–6018
Doody RS, Raman R, Farlow M et al (2013) A phase 3 trial of semagacestat for treatment of Alzheimer disease. New Engl J Med 369(4):341–350
D’Onofrio G, Panza F, Frisardi V et al (2012) Advances in the identification of γ-secretase inhibitors for the treatment of Alzheimer’s disease. Expert Opin Drug Discov 7:19–37
Eikelenboom P, Stam FC (1982) Immunoglobulins and complement factors in senile plaques in Alzheimer’s dementia. Acta Neuropathol 57:239–242
El-Amouri SS, Zhu H, Yu J et al (2008) Neprilysin: an enzyme candidate to slow the progression of Alzheimer’ disease. Am J Pathol 172:1342–1354
Engelhart MJ, Geerlings M, Ruitenberg A et al (2002) Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 287:3223–3229
Eskilinen MH, Kivipelto M (2010) Caffeine as a protective factor in dementia and Alzheimer’s disease. J Alzheimer’s Dis 20(Suppl 1): S167–S174
Fonseca MI, Chu SH, Berci AM et al (2011) Contribution of complement activation pathways to neuropathology differs among mouse models of Alzheimer disease. J Neuroinflamm 8(1):4. doi:10:1186/1742-2094-8-4
Forloni G, Chiesa R, Smiroldo S et al (1993) Apoptosis mediated neurotoxicity induced by chronic application of beta amyloid fragment 25-35. NeuroReport 4:523–526
Frohlich JC (1997) A classification of NSAIDs according to the relative inhibition of cyclooxygenase isoenzymes. TIPS 18:3–34
Fourier A, Letenneur I, Begaud B, Dartigues JF (1996) Nonsteroidal anti-inflammatory drug use and cognitive function in the elderly: inconclusive results from a population-based cohort study. J Clin Epidemiol 49:1201
Games D, Adams D, Allesandrini R et al (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 373:523–527
Gelber RP, Petrovich H, Masaki KH et al (2011) Coffee intake in midlife and risk of dementia and its neuropathological correlates. J Alzheimer’s Dis 23:607–615
Ghosh AK, Brindisi M, Tang J (2012) Developing beta-secretase inhibitors for treatment of Alzheimer’s disease. J Neurochem 120(Suppl 1):71–83
Glenner GG, Wong CW (1984) Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Glenner GG, Wong CW (1984) Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122:1131–1135
Goate A, Chartier-Harlin M-C, Mullan M et al (1991) Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 391:704–706
Goedert M, Wischik RA, Crowther J et al (1988) Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease identification as the microtubule-associated protein tau. Proc Natl Acad Sci USA 85:4051–4055
Goldgaber D, Lerman MI, McBride OW et al (1987) Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science 235:877–880
Griffin WS, Stanley LC, Ling C et al (1989) Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA 86(19):7611–7615
Grundke-Iqbal I, Iqbal K, Tung YC et al (1986) Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:44913–44917
Guerreiro R, Wojtas A, Bras J et al (2013) TREM 2 variants in Alzheimer’s disease. New Engl J Med 368:117–127
Guo J-P, Yu S, McGeer PL (2010) Simple in vitro assays to identify amyloid-beta aggregation blockers for Alzheimer’s disease therapy. J Alzheimers Dis 19:1359–1370
Hardy J, Allsop D (1991) Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12:383–388
Harold D, Abraham R, Hollingworth P et al Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41:1088–1093
Hatashita S, Yamasaki H (2009) Diagnosed mild cognitive impairment due to Alzheimer’s disease with PET biomarkers of beta amyloid and neuronal dysfunction. PloS One. doi: 10.1371/journal.pone/0066877
Hazrati LN, Cauwenberghe C, Brooks PL et al (2012) Genetic association of CR1 with Alzheimer’s disease: a tentative disease mechanism. Neurobiol Aging 33:2949
Heneka MT, Sastre M, Dumitrescu-Ozimek L et al (2005) Acute treatment with the PPAR gamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV171 transgenic mice. Brain 128:1442–1453
Henley DB, May PC, Dean RA, Siemers ER (2009) Development of semegacestat (LY450129), a functional gamma-secretase inhibitor, for the treatment of Alzheimer’s disease. Expert Opin Pharmacother 10:1657–1664
Hillman A, Hahn S, Schilling S et al (2012) No improvement after chronic ibuprofen treatment in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging 33:833
Hippisley-Cox J, Coupland C (2006) Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. BMJ 330:1–7
Holtzman D, Morris JC, Goate AM (2011) Alzheimer’s disease: the challenge of the second century. Sci Transl Med 3(77):1–17
Holmes C, Boche D, Wilkinson D et al (2008) Long term effects of Ab42 immunization in Alzheimer’s disease: follow-up of a randomized, placebo-controlled phase 1 trial. Lancet 372:216–223
Hong L, Koelsch G, Lin X et al (2000) Structure of the protease domain of memapsin 2 (beta-secretase) complexed with inhibitor. Science 290:150–153
Hsiao K, Chapman P, Nilsen S et al (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Ihara Y, Nukina N, Miura R, Ogawara M (1986) Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J Biochem 99:1807–1810
Ikeda S, Allsop D, Glenner GG (1989) Morphology and distribution of plaque and related deposits in the brains of Alzheimer’s disease and control cases. An immunohistochemical study using amyloid beta-protein antibody. Lab Invest 60:113–122
Imbimbo BP, Ottonello S, Frisardi V et al (2012) Solanezumab for the treatment of mild-to-moderate Alzheimer’s disease. Expert Rev Clin Immunol 8:135–149
Int Veldt BA, Ruttenberg A, Hofman A et al (2001) Nonsteroidal anti-inflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med 345:1515–1521
Iwata N, Higuchi M, Saido TC (2005) Metabolism of amyloid-beta peptide and Alzheimer’s disease. Pharmacol Ther 108:129–148
Iwata N, Tsubuki Y, Takaki K et al (2000) Identification of the major A beta (1-42) degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nature Med 6:143–150
Jack CR, Knopman DS, Jagust WJ et al (2010) Hypothetical model of dynamic biomarkers of the Alzheimer pathological cascade. Lancet Neurol 9:119–128
Jantzen PT, Connor KE, diCarlo G et al (2002) Microglial activation and beta amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci 22:2246–2254
Janus C, Pearson J, McLaurin J et al (2000) A beta-peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature 408:970–982
Jonsson T, Stefansson H, Steinberg S et al (2013) Variant of TREM 2 associated with the risk of Alzheimer’s disease. New Engl J Med 368:107–116
Jorm AF, Korten AE, Henderson AS (1987) The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 76:465–470
Kaether C, Haass C, Steiner H (2006) Assembly, trafficking and function of gamma-secretase. Neurodegener Dis 3:275–283
Kakuda N, Akazawa K, Hatsuta H et al (2013) Suspected limited efficacy of γ-secretase inhibitors. Neurobiol Aging 34:1101–1104
Kalback W, Watson MD, Kokjohn TA et al (2002) APP transgenic mice TG2576 accumulate Abeta peptides that are distinct from the chemically modified and insoluble peptides deposited in Alzheimer’s disease senile plaques. Biochemistry 41:922–928
Katzman R (1976) The prevalence and malignancy of Alzheimer’s disease: a major killer. Arch Neurol 33:217–218
Kaufmann WE, Worley PF, Pegg J et al (1996) COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at post synaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA 93:2317–2321
Kim HJ, Chae SC, Lee DK et al (2003) Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J 17:118–120
Klegeris A, Maguire J, McGeer PL (2004) S- but not R-enantiomers of flurbiprofen and ibuprofen reduce human microglial and THP-1 cell toxicity. J Neuroimmunol 152:73–77
Korczyn AD (2008) The amyloid cascade hypothesis. Alzheimer Dementia 4:176–178
Kosik KS, Joachim CL, Selkoe DJ (1986) Microtubule-associated protein tau is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA 83:4044–4048
Kukar T, Murphy MP, Erikson JL et al (2005) Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Aβ 42 production. Nature Med 11:545–550
Kukar T, Prescott S, Erikson JL et al (2007) Chronic administration of R-flurbiprofen attenuates learning impairments in transgenic amyloid precursor mice. BMC Neurosci 8:54
Kuo YM, Kokjohn TA, Beach TG et al (2001) Comparative analysis of amyloid-beta chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer’s disease brains. J Biol Chem 276:12991–12998
Laitenen MH, Ngandu T, Rovio S et al (2006) Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population- based study. Dement Geriatr Cogn Disord 22:99–107
Landi F, Cesari M, Onder G et al (2003) Non-steroidal anti-inflammatory drug use and Alzheimer disease in community-dwelling elderly patients. Am J Geriatr Psychiat 11:179–185
Laporte SL, Bollini SS, Lanz TA et al (2012) Structural basis of C-terminal β-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer’s disease. J Mol Biol 421:525–536
Lee M, Guo JP, Schwab C et al (2012) Selective inhibition of the membrane attack complex of complement by low molecular weight components of the aurin tricarboxylic acid synthetic complex. Neurobiol Aging 33:2237–2246
Leiketsos GG (2007) Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology 68:1800–1808
Leoutsakos JM, Multhen BO, Breitner JC et al (2011) Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline by phase of pre-clinical Alzheimer disease: findings from the randomized control Alzheimer’s disease anti-inflammatory prevention trial. Int J Geriatr Psychiatry. doi:10:1002/gps.2723
Levi-Lahad E, Wasco W, Poorkaj P et al (1995) Candidate gene for the chromosome 1 familial disease locus. Science 269:973–977
Levy E, Carman MD, Fernandez-Madrid IJ et al (1990) Mutation of the Alzheimer’s disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science 248:1124–1126
Li FJ, Shen L, Ji HE (2012) Dietary intakes of vitamin E, vitamin C, and β-carotene and risk of Alzheimer’s disease: a meta-analysis. J Alzheimer’s Dis 31:253–258
Lim GP, Yang F, Chu T et al (2000) Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci 20:5709–5714
Lim JP, Yang P, Chu T et al (2001) Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol Aging 22:983–991
Lindsay J, Laurin D, Verrault R et al (2002) Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol 158:445–453
Lorenzo A, Yankner BA (1994) Beta amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci USA 125:12243–12247
Lourida I, Soni M, Thompson-Coon J et al (2013) Mediterranean diet, cognitive function and dementia: a systemic review. Epidemiology 24:479–489
Ma K, Thomason LA, McLaurin J (2012) Scyllo-inositol, preclinical, and clinical data for Alzheimer disease. Adv Pharmacol 64:177–212
Maia L, de Mendonca A (2002) Does caffeine intake protect from Alzheimer’s disease? Eur J Neurol, pp 377–382
Mangialasche F, Solomon A, Winblad B et al (2011) Alzheimer’s disease: clinical trials and drug development. Lancet Neurol 9:702–716
Masters CL, Simms G, Weinman NA et al (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci 82:4245–4249
Mattsson N, Portelius E, Rolstad S et al (2012) Longitudinal cerebrospinal fluid biomarkers over four years in mild cognitive impairment. J Alzheimer’s Dis 30:767–778
May PC, Gitter BD, Waters DC et al (1992) Beta amyloid peptide in vitro toxicity: lot to lot toxicity. Neurobiol Aging 13:605–607
McGeer PL, Akiyama H, Itagaki S, McGeer EG (1989) Activation of the complement pathway in brain tissue of Alzheimer patients. Neurosci Lett 107:341–346
McGeer PL, Akiyama H, Itagaki S, McGeer EG (1989) Immune response in Alzheimer’s disease. Can J Neurol Sci 16:516–527
McGeer PL, McGeer EG (2003) Is there a future for vaccination as a treatment for Alzheimer’s disease. Neurobiol Aging 24:391–395
McGeer PL, McGeer EG, Rogers J, Sibley J (1990) Anti-inflammatory drugs and Alzheimer disease. Lancet 335:107
McGowan E, Eriksen L, Hutton M (2006) A decade of modeling Alzheimer’s disease in transgenic mice. Trends Genet 22:281–289
McKee AC, Cantu RC, Nowinski CJ et al (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68:2709–2735
McKee AC, Carreras I, Hossain L et al (2008) Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res 1207:225–236
McKee AC, Stein TD, Nowinski CJ et al (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136:43–64
Morgan D, Diamond DM, Gottscall PE et al (2000) Abeta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature 408:982–985
Morihara T, Chu T, Ubeda O et al (2002) Selective inhibition of Aβ42 production by R-enantiomers. J Neurochem 83:1009–1012
Mullard A (2012) String of Alzheimer’s failures offset by upcoming prevention trials. Nature Rev Drug Discov 11:657–660
Naliivaeva NN, Beckett C, Belyaev ND, Turner AJ (2012) Are amyloid-degrading enzymes viable therapeutic targets in Alzheimer’s disease. J Neurochem 120(Suppl 1):167–185
Nicoll JAR, Wilkinson D, Holmes C et al (2003) Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nature Med 9:448–452
Okonko OC, Miellke MM, Griffith HR et al (2011) Cerebrospinal fluid profiles and prospective course and outcome in patients with amnestic mild cognitive impairment. Arch Neurol 68:113–119
Ono K, Condron MM, Teplow DB (2009) Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc Natl Acad Sci USA 106:14745–14750
Orgogozo JM, Gilman S, Dartigues JF et al (2003) Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 61:46–54
Pasaqualletti P, Bonomini C, Dal Forno G et al (2009) A randomized controlled study on the effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin Exp Res 21:102–110
Postina R (2012) Activation of α-secretase cleavage. J Neurochem 120(Suppl 1):46–54
Prestia A, Caroll A, van der Flier WM et al (2013) Prediction of dementia in MCI patients based on core diagnostic markers for Alzheimer disease. Neurology 80(11):1048–1056
Quinn J, Montine T, Morrow J et al (2003) Inflammation and cerebral amyloidosis are disconnected in an animal model of Alzheimer disease. J Neuroimmunol 137:32–41
Reines SA, Block GA, Morris JC et al (2004) Rofecoxib: no effect on Alzheimer’s disease in a 1-year, randomized, blinded, controlled study. Neurology 62:66–71
Robakis NK, Wisniewski HM, Jenkins EC et al (1987) Chromosome 21q21 sublocalization of gene encoding beta-amyloid-peptide in cerebral vessels and neuritic (senile) plaques of people with Alzheimer disease and Down syndrome. Lancet 1:384–385
Rogers J, Cooper NR, Webster S et al (1992) Complement activation by beta-amyloid in Alzheimer’s disease. Proc Natl Acad Sci USA 89:10016–10020
Rogers J, Kirby LC, Hempelman SC et al (1993) Clinical trial of indomethacin in Alzheimer’s disease. Neurology 43:1609–1611
Roses AD (1993) Apolipoprotein E in neurology. Curr Opin Neurol 9(4):265–270
Salloway S, Sperling R, Karen R et al (2011) A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology 77:1253–1262
Sambamurti K, Greig NH, Tadanobu U et al (2011) Targets for AD treatment: conflicting messages from γ-secretase inhibitors. J Neurochem 117:359–374
Scharf S, Mander A, Ugoni A et al (1999) A double blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology 53:197–201
Schenk D, Barbour R, Dunn W et al (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400:173–177
Schneider LS, Lahiri DK (2008) The perils of Alzheimer’s drug development. Curr Alzheimer Res 6:77–78
Schwab C, Hosokawa M, McGeer PL (2004) Transgenic mice overexpressing amyloid beta protein are an incomplete model of Alzheimer disease. Exp Neurol 188:52–64
Selkoe DJ (1991) The molecular pathology of Alzheimer’s disease. Neuron 6:403–409
Shaw LM, Vanderstichele H, Knapik-Czaika M et al (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65(4):403–413
Seppala TT, Nerg O, Koivisto AM et al (2012) CSF biomarkers for Alzheimer disease correlate with brain atrophy findings. Neurology 78(20):11568–11575
Sherrington R, Rogaev EI, Liang Y et al (1995) Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375:754–760
Soininen H, West C, Robbins J, Niculescu L (2007) Long term efficacy and safety of celecoxib in Alzheimer’s disease. Dementia Geriatr Cogn Disord 23:8–21
Sperling RA, Aisen PS, Beckett LA et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia, pp 280–292
Spillantini MG, Goedert M (2013) Tau pathology and neurodegeneration. Lancet Neurol 12:609–622
Steinerman JR, Irizarry M, Scarmeas N et al (2008) Distinct pools of beta-amyloid in Alzheimer’s disease-affected brain: a clinicopathologic study. Arch Neurol 65:906–912
Stelzman RA, Schnitlen HN, Murtagh FR (1995) An English translation of Alzheimer’s 1907 paper. Clin Anat 8:429–431
Stewart WF, Klawas C, Corrada M, Metter EJ (1997) Risk of Alzheimer disease and duration of NSAID use. Neurology 48:626–632
Sung S, Yang Y, Uryu K et al (2004) Modulation of nuclear factor κB activity by indomethacin influences Aβ levels but not Aβ precursor protein metabolism in a model of Alzheimer’s disease. Am J Pathol 165:2197–2206
Szekely CA, Green RC, Breitner JH (2008) No advantage of Aβ42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology 70:2291–2298
Tayeb HO, Murray ED, Price BH, Tarazi FI (2013) Bapineuzamab and solanezumab for Alzheimer’s disease: is the amyloid cascade hypothesis still alive? Expert Opin Biol Ther 13:1075–1084
Theis M, Bleiler S (2013) Alzheimer’s disease facts and figures. Alzheimer’s Dem 9:208–245
Toledo JB, Shaw LM, Trojanowski JQ (2013) Plasma amyloid beta measurements: a desired but elusive Alzheimer’s disease marker. Alzheimer’s Res Ther 5:8
Van Broeckhoven C, Haan J, Bakker E et al (1990) Amyloid beta protein precursor gene and hereditary cerebral hemorrhage, Dutch type. Science 248:1120–1122
Van Dam D, Coen K, de Deyn PP (2010) Ibuprofen modifies cognitive disease progression in an Alzheimer’s mouse model. J Psychopharmacol 24:383–388
Van Groen T, Kadish I (2005) Transgenic model mice, effects of potential AD treatments on inflammation and pathology. Brain Res Rev 48:370–378
Van Groen T, Miettinen P, Kadish I (2011) Transgenic AD model mice, effects of potential anti-AD treatments on inflammation. J Alzheimer’s Dis 24:301–313
Vassar R, Bennett BD, Babu-Khan S et al (1999) Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735–741
Villemagne VL, Burnham S, Bourgeat P et al (2013) Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. doi:10.1016/S1474-4442(13)70044-9
Visser PJ, Verhey F, Knol DL et al (2009) Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol 8:619–627
Vlad SC, Miller DR, Kowall NW, Felson DT (2008) Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 70:1672–1677
Vlassenko AG, Mintun MA, Xiong C et al (2011) Amyloid-beta plaque growth in cognitively normal adults: longitudinal [11C] Pittsburgh compound B data. Ann Neurol 70(5):857–861
Wang PY, Chen JJ, Su HM (2010) Docosohexaenoic acid supplementation of primary hippocampal neurons attenuates the neurotoxicity induced by aggregated amyloid beta protein(42) and up-regulates cytoskeletal protein expression. J Nutr Biochem 31:345–350
Webster SD, Lue F, Brachova L et al (1997) Molecular and cellular characterization of the membrane attack complex C5b-9 in Alzheimer’s disease. Neurobiol Aging 18:415–421
Webster SD, Tenner AJ, Paulos TL et al (1999) The mouse C1q A-chain sequence alters beta-amyloid–induced complement activation. Neurobiol Aging 18:415–421
Weggen S, Erikson JL, Das P et al (2001) A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414:212–216
Wisniewski HM, Iqbal K, Banchewr C et al (1989) Cytoskeletal pathology and the formation of beta-amyloid fibers in Alzheimer’s disease. Neurobiol Aging 10:409–414
Wisniewski T, Sadowski M (2008) Preventing β-amyloid fibrillization and deposition: β-sheet breakers and pathological chaperone inhibitors. BMC Neurosci 9(Suppl 2):55
Wolfe MS (2012) γ-Secretase as a target for Alzheimer’s disease. Adv Pharmacol 64:127–153
Wolfson C, Perrault A, Moride Y et al (2002) A case-control analysis of nonsteroidal anti-inflammatory drugs and Alzheimer disease: are they protective? Neuroepidemiology 21:81–86
Wyss-Coray T, Yan F, Lin AH et al (2009) Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci USA, pp 10837–10842
Xia W, Yang T, Shankar G et al (2009) A specific enzyme-linked immunosorbent assay for measuring beta-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch Neurol 66:190–199
Yan Q, Zhang J, Liu H et al (2003) Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci 23:7504–7509
Yankner BA, Dawes LR, Fisher S et al (1989) Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer’s disease. Science 245:417–420
Yasojima K, McGeer EG, McGeer PL (2001) Relationship between beta amyloid peptide generation molecules and neprilysin in Alzheimer disease and normal brain. Brain Res 919:115–121
Yip AG, Green RC, Huyck M et al (2005) Non steroidal anti-inflammatory drug use and Alzheimer’s disease risk: the Mirage study. BMC Geriatr 5:2
Zanjani H, Finch CE, Kemper C et al (2005) Complement activation in very early Alzheimer disease. Alzheimer Dis Assoc Disord 19:55–56
Zandi PP, Anthony JC, Hayden KM et al (2002) Reduced incidence of AD with NSAID but not H2 receptor antagonists. The Cache County study. Neurology 59:880–886
Zetterberg H, Pedersen M, Lind K et al (2007) Intra-individual stability of CSF biomarkers for Alzheimer’s disease over two years. J Alzheimer’s Dis 12:255–260
Zhou J, Fonseca MI, Pisalyaput K, Tenner AJ (2008) Complement C3 and C4 expression in C1q sufficient and deficient mouse models of Alzheimer’s disease. J Neurochem 106:2080–2092
Acknowledgments
This work was supported by contributions from individual British Columbians. The authors declare they are shareholders in Aurin Biotech Inc., a startup biotechnology company with a mandate to develop complement inhibitors to treat human diseases.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McGeer, P.L., McGeer, E.G. The amyloid cascade-inflammatory hypothesis of Alzheimer disease: implications for therapy. Acta Neuropathol 126, 479–497 (2013). https://doi.org/10.1007/s00401-013-1177-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-013-1177-7