Abstract
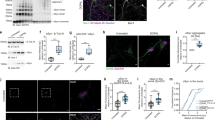
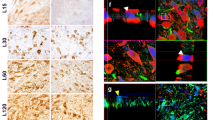
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the selective loss of dopamine (DA) neurons and the presence of α-synuclein (AS) aggregates as Lewy bodies (LBs) in the remaining substantia nigra (SN) neurons. A continuing puzzle in studying PD pathogenesis is that although AS is expressed throughout the brain, LBs and selective dopaminergic cell loss lead to characteristic clinical signs of PD, suggesting that there is a link between AS aggregation and DA metabolism. One potential candidate for this link is the monoamine oxidase (MAO) metabolite of DA, 3,4-dihydroxyphenylacetaldehyde (DOPAL), as neither DA nor DA metabolites other than DOPAL are toxic to SN neurons at physiological concentrations. We tested DOPAL-induced AS aggregation in a cell-free system, in vitro in DA neuron cultures and in vivo with stereotactic injections into the SN of Sprague–Dawley rats by Western blots, fluorescent confocal microscopy and immunohistochemistry. We demonstrate that DOPAL in physiologically relevant concentrations, triggers AS aggregation in the cell-free system, and in cell cultures resulting in the formation of potentially toxic AS oligomers and aggregates. Furthermore, DOPAL injection into the SN of Sprague–Dawley rats resulted in DA neuron loss and the accumulation of high molecular weight oligomers of AS detected by Western blot. Our findings support the hypothesis that DA metabolism via DOPAL can cause both DA neuron loss and AS aggregation observed in PD.






Similar content being viewed by others
Abbreviations
- PD:
-
Parkinson’s disease
- DA:
-
Dopamine
- DAQ:
-
Dopamine quinone
- CA:
-
Catecholamine
- AS:
-
α-synuclein
- LB:
-
Lewy bodies
- MAO:
-
Monamine oxidase
- mPTP:
-
Mitochondrial permeability transition pore
- DOPAL:
-
3,4-Dihydroxyphenylacetaldehyde
- SN:
-
Substantia nigra
- PVDF:
-
Polyvinilidene difluoride
- TBS:
-
Tris buffered saline
- PCR:
-
Polymerase chain reaction
- 5Y:
-
SHSY-5Y cells
- ECL:
-
Enhanced chemiluminescence
- TH:
-
Tyrosine hydroxylase
- DAB:
-
Diamonobenzidine dichloride
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- HVA:
-
Homovanillic acid
- WT:
-
Wild type
- IR:
-
Immunoreactivity
- DAT:
-
Dopamine transporter
- ALDH:
-
Aldehyde dehydrogenase
- PAGE:
-
Polyacrylamide gel electrophoresis
- VTA:
-
Ventral tegmental area
References
Arasate M, Mitra S, Schweitzer ES, Segal MR (2004) Inclusion body formation reduces levels of mutant huntingtin and risk of neuronal death. Nature 431:805–810
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces feature of Parkinson’s disease. Nature Neurosci 3:1301–1306
Blashko H (1952) Amine oxidase and amine metabolism. Pharmacol Rev 4:415–453
Burke WJ (2003) 3,4-Dihydroxyphenylacetaldehyde: a potential target for neuroprotective therapy in Parkinson’s disease. Current Drug Targets. CNS Neurol Disord 2:143–148
Burke WJ, Li SW, Anwar M, Glickstein SB, Ruggiero DA (2001) Catecholamine-derived aldehyde induces apoptosis in adrenergic neurons in rostral ventral lateral medulla. Brain Res 891:218–227
Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Zahm DS (2004) Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology 25:101–115
Burke WJ, Li SW, Williams EA, Nonneman R, Zahm DS (2003) Dihydroxyphenylacetaldehyde is the toxic dopamine metabolite in vivo: implications for Parkinson’s disease pathogenesis. Brain Res 989:205–213
Burke WJ, Schmitt CA, Gillespie KN, Li SW (1996) 3,4-Dihydroxyphenylglycolaldehyde, the MAO-A metabolite of norepinephrine, is selectively toxic to differentiated rat pheochromocytoma cells. Brain Res 772:232–235
Cappai R, leck S-L, Tew DJ, Williamson NA, Smith DP, Galatis D, Sharples RA, Curtain CC, Ali FE, Cherney RA, Culvenor JG, Bottomley SP, Masters CL, Barnham KJ, Hill AF (2005) Dopamine promotes α-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J 19: 1377–1396
Coleman P, Federoff H, Kurlan R (2004) A focus on the synapse for neuroprotection in Alzheimer’s disease and other dementias. Neurology 63:1155–1162
Conway KA, Rochet JC, Bieganski RM, Lansbury PT (2001) Kinetic stabilization of the α-synuclein protofibril by a dopamine α-synuclein adduct. Science 292:1346–1349
Dawson TM, Dawson VL (2003) Molecular pathways of degeneration in Parkinson’s disease. Science 302:819–821
Fahn S (1997) Levo-dopa-induced neurotoxicity. CNS Drugs 8:276–393
Filloux F, Townsend JJ (1993) Pre- and postsynaptic neurotoxic effects demonstrated by intrastriatal injection. Exp Neurol 119:79–88
Galvin JE (2006) Interaction of alpha-synuclein and dopamine metabolites in pathogenesis of Parkinson’s disease: a case for selective vulnerability of substantia nigra. Acta Neuropathol 112:115–126
Galvin JE, Lee VM, Trojanowski JD (2001) Synucleinopathies: clinical and pathological implications. Arch Neurol 58:186–190
Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch FE, Krafft GA, Klein WL (2003) Alzheimer’s disease-affected brain: presence of oligomeric AB ligands (ADDLS) suggest a molecular basis for reversible memory loss. Proc Natl Acad Sci USA 100:10417–10422
Greenamyre JT, Hastings TG (2004) Parkinson’s—divergent causes, convergent mechanisms. Science 304:1120–1122
Hashimoto M, Hsu LJ, Sisk A, Xia Y, Taked A, Sundmo M, Masliah E (1998) Human recombinant NACP/α-synuclein is aggregated and fibrillated in vitro: relevance for Lewy body disease. Brain Res 99:301–306
Hirsch EC, Faucheux B, Damier P, Mouatt-Prigent A, Agid Y (1997) Neuronal vulnerability in Parkinson’s disease. J Neural Transm 50:79–88
Hornykiewicz O (1996) Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev 18:925–965
Jellinger K (1987) Overview of morphological changes in Parkinson’s disease. J Adv Neurol 45:1–18
Kristal BS, Conway AD, Brown AM, Jain JC, Ulluci PA, Li SW, Burke WJ (2000) Selective dopaminergic vulnerability: 3,4-dihydroxyphenylacetaldehyde targets mitochondria. Free Radic Bio Med 30:24–931
Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT (1998) Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nature Genet 18:106–108
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola K (1998) Diffusible, nonfibrillar ligands derived from AB 1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95:6448–6453
Lamensdorf I, Eisenhofer G, Harvery-White J, Nechustan A, Kirk K, Kopin IJ (2000) Dihydroxyphenylacetaldehyde potentiates the toxic effects of metabolic stress in PC 12 cells. Brain Res 868:191
Lee FJS, Liu F, Pristupa ZB, Niznik HB (2001) Direct binding and functional coupling of α-synuclein to dopamine transporters accelerate dopamine-induced apoptosis. FASEB J 15:916–926
Li HT, Lin DH, Luo XY, Zhang F, Ji LN, Du HN, Hi J, Zhou JW, Hu HY (2005) Inhibition of α-synuclein fibrillation by dopamine analogs via reaction with amino groups of α-synuclein: Implication for dopaminergic neurodegeneration. FEBS J 272:3661–3672
Li SW, Burke WJ (1998) Synthesis of a biochemically important aldehyde, 3,4-dihydroxyphenylacetaldehyde. Bioorg Chem 26:45–50
Li SW, Li HT, Minteer S, Burke WJ (2001) 3,4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson’s disease pathogenesis. Brain Res 93:1–7
Mandel S, Grunblatt E, Riederer P, Amariglio N, Hirsch JJ, Rechari G, Youdim MBH (2007) Gene expression profiling of sporadic Parkinson’s disease substantia nigra pars compacta reveals impairment of ubiquitin proteosome subunits, SKP1A, aldehyde dehydrogenase, and chaperone HSC-70. Ann NY Acad Sci 1053:356–375
Mazzulli JR, Mishizen AJ, Giasson BJ, Lynch DR, Thomas SA, Nakashima A, Nagatsu T, Ota A, Ischiropoulos H (2006) Cytosolic catechols inhibit α-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci 26:10068–10078
Miller DW, Hague SM, Clairmon J, Baptista M, Gwinn-Hardy K, Cokson MR, Singleton AB (2004) Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology 62:1835–1838
Nagatsu T, Sawada M (2006) Cellular and molecular mechanisms of Parkinson’s disease: neurotoxins, causative genes and inflamatory cytokines. Cell Mol Neurobiol 26:779–800
Narhi L (1999) Both familial Parkinson’s disease mutations accelerate α-synuclein aggregation. J Biol Chem 274:9843–9846
Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, Lee VM (2005) Reversible inhibition of α-synuclein fibrillizationation by dopaminochrome-mediated conformational alterations. J Biol Chem 280:21212–21219
Osterova-Golts N, Petrucelli L, Hardy J, Lee JM, Farer M, Wolozin B (2000) The A53T α-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci 20:6048–6054
Paik SR, Shin HJ, Lee JH (2000) Metal catalyzes oxidation of α-synuclein in the presence of copper (II) and hydrogen peroxide. Arch Biochem Biophys 378:269–277
Pandey N, Schmidt RE, Galvin JE (2006) Mutations of KTKEGV repeat region of alpha-synuclein promotes aggregation in cultured cells. Expl Neurol 187:1515–1520
Parkinson J (1817) An Essay on Shaking Palsy
Polymeropoulos MD, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Stenroos ES, Chandraskharappa s, Athanassiadou H, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Dilorio G, Golbe LI, Nussbaum RL (1997) Mutation in α-synuclein gene identified in families with Parkinson’s disease. Science 275:2045–2047
Sawada H, Khono R, Kihara T, Izumi Y, Sakka N, Ibi M, Nakanishi M, Nakamizo T, Yamakawa K, Shibasaki H (2004) Proteasome mediates dopaminergic neuronal degeneragion and its inhibition causes alpha-synuclein inclusions. J Biol Chem 279:10710–10719
Schapira AHV, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54:823–827
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goerdert M (1997) α-Synuclein in Lewy bodies. Nature 388:839–840
Swerdlow RH, Parks JK, Davis JN, Cassarino BS, Trimmer PA, Currie LJ, Dougherty S, Bridges WS, Bennet JP, Wooten GF, Parker DW (2004) Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson’s disease family. Ann Neurol 44:873–881
Volles MJ, Lansbury PT (2002) Vesicle permeabilization by protofibrillar α-synuclein: comparison of wild type with Parkinson’s disease-linked mutants and insights in the mechanism. Biochemistry 41:4595–4602
Volles MJ, Lee SJ, Rochet JC, Schtelerman MD, Ding JT, Kessler JC, Lansbury PT (2001) Vesicle permeabilization by protofibrillar α-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry 40:7812–7819
Xu J, Kao SY, Song W, Jin LW, Yankner BA (2002) Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson’s disease. Nat Med 8:600–606
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros RB, Ampuero I, Vidal L, Hoeniaka J, Rodríguez O, Atares B (2004) The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol 55:164–173
Acknowledgments
The authors are grateful for the help of Prof. Vijaya Lakshmi and Mrs. Priscilla Dehaven in some of the experiments. They also thank Dr. John Trojanowski for the AS 202 antibody. This study was supported by grants from Missouri ADRDA Program (WJB, NP), Nestle Foundation (VBK), St Louis VAMC (WJB,VBK), NIH HL 64772 (WMP), NIH AG20764, AG 03991, AG 05681 (JEG), the American Federation on Aging Research (JEG), and generous gifts from the Alan A. and Edith L. Wolff Charitable Trust (JEG) and Blue Gator Foundation (JEG). Dr. Galvin is a recipient of the Paul Beeson Physician Faculty Scholar Award in Aging Research and the American Academy of Neurology Research Award in Geriatric Neurology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from Missouri ADRDA Program (WJB, NP), Nestle Foundation (VBK), St. Louis VAMC (WJB,VBK), NIH HL 64772 (WMP), NIH AG20764, AG 03991, AG 05681 (JEG), the American Federation on Aging Research (JEG), and generous gifts from the Alan A. and Edith L. Wolff Charitable Trust (JEG) and Blue Gator Foundation (JEG).
Rights and permissions
About this article
Cite this article
Burke, W.J., Kumar, V.B., Pandey, N. et al. Aggregation of α-synuclein by DOPAL, the monoamine oxidase metabolite of dopamine. Acta Neuropathol 115, 193–203 (2008). https://doi.org/10.1007/s00401-007-0303-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-007-0303-9