Abstract
B-type natriuretic peptide (BNP) and C-type natriuretic peptide (CNP), and (Cys-18)-atrial natriuretic factor (4–23) amide (C-ANF), are cytoprotective under conditions of ischemia–reperfusion, limiting infarct size. ATP-sensitive K+ channel (KATP) opening is also cardioprotective, and although the KATP activation is implicated in the regulation of cardiac natriuretic peptide release, no studies have directly examined the effects of natriuretic peptides on cardiac KATP activity. Normoxic cardiomyocytes were patch clamped in the cell-attached configuration to examine sarcolemmal KATP (sKATP) activity. The KATP opener pinacidil (200 μM) increased the open probability of the patch (NPo; values normalized to control) at least twofold above basal value, and this effect was abolished by HMR1098 10 μM, a selective KATP blocker (5.23 ± 1.20 versus 0.89 ± 0.18; P < 0.001). We then examined the effects of BNP, CNP, C-ANF and 8Br-cGMP on the sKATP current. Bath application of BNP (≥10 nM) or CNP (≥0.01 nM) suppressed basal NPo (BNP: 1.00 versus 0.56 ± 0.09 at 10 nM, P < 0.001; CNP: 1.0 versus 0.45 ± 0.16, at 0.01 nM, P < 0.05) and also abolished the pinacidil-activated current at concentrations ≥10 nM. C-ANF (≥10 nM) enhanced KATP activity (1.00 versus 3.85 ± 1.13, at 100 nM, P < 0.05). The cGMP analog 8Br-cGMP 10 nM dampened the pinacidil-activated current (2.92 ± 0.60 versus 1.53 ± 0.32; P < 0.05). Natriuretic peptides modulate sKATP current in ventricular cardiomyocytes. This may be at least partially associated with their ability to augment intracellular cGMP concentrations via NPR-A/B, or their ability to bind NPR-C with high affinity. Although the mechanism of modulation requires elucidation, these preliminary data give new insights into the relationship between natriuretic peptide signaling and sKATP in the myocardium.
Similar content being viewed by others
Introduction
The natriuretic peptides are a family of structurally related mediators with diverse autocrine/paracrine and endocrine functions in multiple tissues but they are especially involved in cardiovascular homeostasis [6, 31]. In the circulation, C-type natriuretic peptide (CNP), which is predominantly of vascular origin under normal physiological conditions, exerts autocrine/paracrine actions that are well characterized in the vessel wall [33]. The cardiac-derived atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) exert pressure- and volume-regulating roles, which may be viewed as classic endocrine functions [31]. However, there is extensive evidence that ANP and BNP also exert multiple autocrine/paracrine actions within cardiac tissue [6]. These local cardiac actions may be particularly important under pathological conditions when there is enhanced release of ANP and BNP from tissue stores [40]. These include conditions associated with pressure or volume overload, cardiac remodeling and hypoxia where the peptides may exert fundamental (counter-) regulatory actions within myocardium [17, 28, 40, 45].
Limited pharmacological evidence suggests a role of ATP-sensitive K+ channel (KATP) opening since protection is lost in the presence of the channel blockers glibenclamide and sodium 5-hydroxydecanoate [5]. This latter mechanism is little understood. Of particular interest, in pancreatic islet β cells, ANP exerts an insulinotrophic action, associated with KATP blockade [36, 43]. Furthermore, inwardly rectifying K+ channel 6.2 (Kir6.2) deficient mice were demonstrated to be more susceptible to stretch-induced ANP release compared to wild type, suggesting a negative feedback axis between KATP and cardiac natriuretic peptide release [38]. These findings are intriguing as they suggest a plausible regulatory relationship between natriuretic peptides and KATP in distinct endocrine secretory glands and specialized endocrine organs [36, 38]. It is noteworthy that both cardiac and pancreatic KATP contain the Kir6.2 core. However, they differ with respect to the sulphonylurea receptor (SUR), with Kir6.2 coupled to SUR2A in cardiomyocytes and to SUR1 in pancreatic beta cells 1:1 tetrameric stoichiometry [1, 39].
In view of the increasing interest in the roles and therapeutic potential of natriuretic peptides in cardiac disease, it is important to characterize the actions of natriuretic peptides on KATP function in cardiomyocytes. As such, this study provides the first comprehensive and comparative electrophysiological investigation of natriuretic peptides on cardiac sarcolemmal KATP (sKATP) activity. After characterizing sKATP activity in adult rat ventricular cardiomyocytes, we sought to test the hypothesis that natriuretic peptides promote sKATP opening by observing the effects of BNP and CNP together with the natriuretic peptide clearance receptor (NPR-C) agonist (Cys-18)-atrial natriuretic factor (4–23) amide (C-ANF) on sKATP activity in these cells. Our data provide a characterization of the actions of natriuretic peptides on sKATP. They strongly suggest that, rather than activating sKATP, BNP and CNP at physiological concentrations, and at supraphysiological concentrations relevant to circulating plasma levels in cardiac disease and therapeutic use, inhibit the ion channel. They also suggest that the inhibition seen with BNP and CNP is not due to NPR-C agonism because C-ANF did not depress sKATP activity.
Methods
Cardiomyocyte isolation
We used a total of 64 adult male Sprague–Dawley rats (270–350 g, Harlan Laboratories Bicester, Oxford) for this study. Their care and use were in accordance with UK Home Office Guidelines on the Animals (Scientific Procedures) Act 1986 (The Stationary Office, London, UK). Following pentobarbital anesthesia, hearts were excised and left ventricular cardiac myocytes were isolated using a standard enzymatic digestion protocol. Myocytes were seeded at a density of 20,000 rods/well on extracellular matrix gel-coated plastic coverslips, and cultured overnight under normal CO2 incubator conditions at 37 °C, prior to treatments and patch clamping. See online resource for full details.
Electrophysiology
The bath solution was in mM: 150 NaCl, 3 KCl, 10 d-glucose, 10 HEPES, pH 7.2. The recording pipette contained in mM: 5 NaCl, 140 KCl, 1 MgCl2, 1 CaCl2, 11 EGTA, 10 HEPES, pH 7.2. During the sKATP channel characterization phase of this study (see series 1), pipette solutions containing KCl 70 mM:NaCl 70 mM (NaCl, 70 mM equimolar substitution) and KCl 200 mM were used as comparator to the standard pipette solution. An Axon CV-4 patch clamp headstage (Axon Instruments, USA) was mounted on a three axis hydraulic micromanipulator (Narashige, Japan). Signals were amplified using an Axopatch 1D patch clamp amplifier (Axon Instruments, USA) and Neurolog DC amplifier (Digitimer Ltd., UK), and digitized using a National Instruments BNC 2110 digitizer. Signals were typically filtered at 5 kHz and sampling rate was 20 kHz. Signals were visualized on an OX722 METRIX oscilloscope (ITT instruments) or computer screen.
Electrodes were pulled from filamented borosilicate glass capillaries (1.5/0.86 OD and ID, respectively; Harvard Apparatus, UK) and fine polished using a DMZ Universal Puller (Zeitz-Instrumente, Germany). Microelectrodes had resistances of 5–10 MΩ.
Single channel recordings were made from cell-attached patches, and performed at room temperature 22–24 °C, as our setup does not contain a Peltier thermoelectric device for cooling and heating. The electrophysiological gating properties of adult rat cardiac KATP do not significantly change at temperatures ranging 20–30 °C [26]. In an independent study, Kohlhardt and colleagues observed a consistent but slight increase in neonatal rat cardiac KATP activity at temperatures ranging 29–39 °C compared to 19–29 °C [21].
Following gigaohm seal formation, currents passing through single ion channels were observed and recorded. Recordings (45 s) were made over a range of patch potentials: 0, −30, −60, −90, and −120 mV. The parameter NPo, where N is the number of channels in the patch and Po the open probability of one channel, was used to determine the effects of different compounds and natriuretic peptides on KATP activity. Po is derived from the sum of individual channel opening times (O) and individual closed times (C), thus Po = O/(O + C). WinEDR v3.2.2 software (Strathclyde University, UK) was used for data acquisition and single channel analysis.
Materials
Rat BNP-(1–32) and C-ANF-(4–23) were obtained from Sigma-Aldrich UK, and both CNP-(1–22) and 8Br-cGMP from Tocris bioscience UK. sKATP channel opener pinacidil (Sigma-Aldrich, UK) was dissolved in dimethylsulfoxide (DMSO; maximal final concentration of 0.25 % v/v). HMR1098, a selective sKATP inhibitor, was the kind gift of Dr Jürgen Pünter, Sanofi-Aventis Germany. With the exception of HMR1098, all compounds were diluted in bath solution (see recording solutions); HMR1098 was diluted in unsupplemented medium 199.
Treatments
The number of cells patched is shown in brackets. All cells from group 4 onwards were patched with KCl 140 mM in the patch pipette. All treatments were randomized.
Series 1
In these experiments, we sought to characterize the ion selectivity and conductance of sKATP, thus cells were patched using different concentrations of KCl with or in the absence of NaCl, which was used as an equimolar substitute (group 1–3). In addition, long established and experimentally characterized KATP modulators were used to pharmacologically test whether the channel observed in our patch clamp recordings is sKATP (group 4–7).
Ion selectivity experiments
Group 1, KCl 70 mM and NaCl 70 mM (n = 4)
Group 2, KCl 140 mM (n = 5)
Group 3, KCl 200 mM (n = 4)
sKATP channel modulation experiments
Group 4, control (n = 12): cells were pretreated with unsupplemented medium 199 for 30 min, then patch clamped in bath solution, or in bath solution containing DMSO 0.25 % v/v.
Group 5, pinacidil 200 μM (n = 7): cells were pretreated with unsupplemented medium 199 for 30 min, then patch clamped following bath application of pinacidil.
Group 6, HMR1098 10 μM (n = 8): cells were pretreated with HMR1098 in unsupplemented medium 199 for 30 min, then patch clamped in bath solution, or in bath solution containing DMSO 0.25 % v/v.
Group 7, HMR1098 + pinacidil (n = 6): cells were pretreated with HMR1098 in unsupplemented medium 199 for 30 min, then patch clamped following bath application of pinacidil.
Series 2
These experiments were designed to examine the effect of natriuretic peptides on sKATP channel activity and conductance. Cells were patched clamped in bath solution containing BNP, CNP or C-ANF, in the absence or in the presence of pinacidil. All natriuretic peptides were applied at six concentrations ranging from 0.01 to 1,000 nM. Two independent sets of experiments were done for low concentrations (0.01, 0.1 and 1 nM) and high concentrations (10, 100 and 1,000 nM) of BNP and CNP, with each series having their own separate control and pinacidil treatment groups, respectively. Experiments with C-ANF were done as a single set.
The effect of BNP on sKATP activity
Set 1: the effect of low concentrations of BNP on sKATP activity
Group 8, control (n = 8): cells were patched clamped in bath solution, or in bath solution containing DMSO 0.25 % v/v.
The following compounds were bath applied:
Group 9, pinacidil 200 μM (n = 10)
Group 10, BNP 0.01 nM (n = 5)
Group 11, BNP 0.1 nM (n = 3)
Group 12, BNP 1 nM (n = 5)
Group 13, BNP 0.01 nM + pinacidil (n = 3)
Group 14, BNP 0.1 nM + pinacidil (n = 5)
Group 15, BNP 1 nM + pinacidil (n = 3)
Set 2: the effect of high concentrations of BNP on sKATP activity
Group 16, control (n = 35): cells were patched clamped in bath solution, or in bath solution containing DMSO 0.25 % v/v.
The following compounds were bath applied:
Group 17, pinacidil 200 μM (n = 41)
Group 18, BNP 10 nM (n = 9)
Group 19, BNP 100 nM (n = 8)
Group 20, BNP 1,000 nM (n = 14)
Group 21, BNP 10 nM + pinacidil (n = 9)
Group 22, BNP 100 nM + pinacidil (n = 8)
Group 23, BNP 1,000 nM + pinacidil (n = 10)
The effect of CNP on sKATP activity
Set 1: the effect of low concentrations of CNP on sKATP activity
Group 24, control (n = 5): cells were patched clamped in bath solution, or in bath solution containing DMSO 0.25 % v/v.
The following compounds were bath applied:
Group 25, pinacidil 200 μM (n = 4)
Group 26, CNP 0.01 nM (n = 3)
Group 27, CNP 0.1 nM (n = 3)
Group 28, CNP 1 nM (n = 3)
Group 29, CNP 0.01 nM + pinacidil (n = 4)
Group 30, CNP 0.1 nM + pinacidil (n = 3)
Group 31, CNP 1 nM + pinacidil (n = 4)
Set 2: the effect of high concentrations of CNP on sKATP activity
Group 32, control (n = 29): cells were patched clamped in bath solution, or in bath solution containing DMSO 0.25 % v/v.
The following compounds were bath applied:
Group 33, pinacidil 200 M (n = 34)
Group 34, CNP 10 nM (n = 10)
Group 35, CNP 100 nM (n = 9)
Group 36, CNP 1,000 nM (n = 16)
Group 37, CNP 10 nM + pinacidil (n = 8)
Group 38, CNP 100 nM + pinacidil (n = 8)
Group 39, CNP 1,000 nM + pinacidil (n = 8)
The effect of low and high concentrations of C-ANF on sKATP activity
Group 40, control (n = 7): cells were patched clamped in bath solution, or in bath solution containing DMSO 0.25 % v/v.
The following compounds were bath applied:
Group 41, pinacidil 200 μM (n = 11)
Group 42, C-ANF 0.01 nM (n = 6)
Group 43, C-ANF 0.1 nM (n = 3)
Group 44, C-ANF 1 nM (n = 5)
Group 45, C-ANF 0.01 nM + pinacidil (n = 7)
Group 46, C-ANF 0.1 nM + pinacidil (n = 4)
Group 47, C-ANF 1 nM + pinacidil (n = 7)
Group 48, C-ANF 10 nM (n = 5)
Group 49, C-ANF 100 nM (n = 4)
Group 50, C-ANF 1,000 nM (n = 5)
Group 51, C-ANF 10 nM + pinacidil (n = 4)
Group 52, C-ANF 100 nM + pinacidil (n = 4)
Group 53, C-ANF 1,000 nM + pinacidil (n = 6)
Series 3
cGMP generation is the common second messenger signal following receptor stimulation by BNP and CNP. These experiments examined if 8Br-cGMP, a cell-permeable analog of cGMP, could mimic the effects of these peptides.
The effect of cGMP on sKATP activity
Group 54, control (n = 18): cells were patched clamped in bath solution, or in bath solution containing DMSO 0.25 % v/v.
The following compounds were bath applied:
Group 55, pinacidil 200 μM (n = 12)
Group 56, 8Br-cGMP 10 nM (n = 10)
Group 57, 8Br-cGMP + pinacidil (n = 14)
PCR and western blotting
Gene and protein expression of KATP channel subunits were determined in myocardial tissue extracts by RT-PCR and Western blotting. See online resource for full descriptions.
Data analysis
Data are expressed as mean ± standard error of the mean (SEM). Ion channel open probabilities (NPo) are normalized to control. Raw data corresponding to specific treatment groups were compared for statistical significance using Dunnett’s or Newman-Keuls’ multiple comparison tests following one-way analysis of variance (ANOVA). Differences between arithmetic means were considered significant when P < 0.05. Data were analyzed using GraphPad Prism 5 software (GraphPad software Inc., USA).
Results
sKATP channel composition revealed by PCR and Western blotting
We confirmed the expression of all KATP subunits at the gene and protein level (Figs. 1, 2) in at least three out of four ventricular myocardial samples analyzed. Strong gene expression was evident for all subunits in all samples analyzed; furthermore, Kir6.1, Kir6.2, SUR1 and SUR2 subunit proteins were strongly expressed. These expression patterns confirm the presence of all KATP subunit proteins in the cardiomyocyte and indicate the possibility that alternative KATP subunit configurations might be present in the cardiac sarcolemma alongside the native Kir6.2/SUR2A channel.
RT PCR amplification of GAPDH and KATP pore forming and receptor subunit mRNA. Samples are from four independent cardiomyocyte isolations from rat left ventricle. The gene coding for each KATP subunit is clearly seen. All samples were diluted in Novel Juice (Genedirex, USA), a non-mutagenic nucleic acid stain, and were separated on the same 15 by 25 cm 1 % agarose gel for 6 h prior to UV transillumination and photo aquisition. The following PCR products were obtained, and the gene and product size is shown in brackets: GAPDH (223 bp), Kir6.1 (KNCJ8, 227 bp), Kir6.2 (KNCJ11, 201 bp), SUR1 (ABCC8, 169 bp) and SUR2 (ABCC9, 228 bp)
Western blots showing the protein expression of the KATP pore forming and receptor subunits in cardiomyocytes isolated from left ventricle. Samples consist of protein extracted from the same four independent cardiomyocyte isolations as mentioned in the legend for Fig. 1. Strong Kir6.2, SUR1 and SUR2 protein expression is seen, whereas Kir6.1 expression is weak comparably. A 70 kDa band is seen for SUR1 and not the predicted 177 kDa, but according to Pu and colleagues [32], this could be a SUR1 short form splice variant. The following amount of protein was loaded when probing for each KATP subunit: 150 μg for Kir6.1, 80 μg for Kir6.2, and 100 μg for SUR1 and SUR2
sKATP electrophysiological characterization
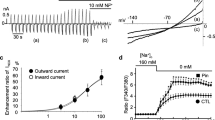
Differing sKATP openings, unitary currents and conductance states were observed in cell-attached patches for each patch pipette configuration (Fig. 3a, b). Changes in KCl concentration (70, 140 and 200 mM) and interpolation of current voltage relationships yielded unitary conductances of 44.03 ± 1.38, 54.77 ± 1.83, and 61.31 ± 1.87 pS. An increase in unitary conductance was seen when the concentration of KCl in the patch pipette was increased, and the changes observed were statistically significant (44.03 ± 1.38 versus 54.77 ± 1.83 pS, P < 0.01; 61.31 ± 1.87 versus 54.77 ± 1.83 pS, P < 0.05). The intermediary unitary conductances are probably indicative of a functional chimeric sKATP with likely co-assembled pore forming subunits of Kir6.1 and 6.2 coupled with SUR2A [12].
Representative sKATP recordings (a and c), current–voltage plots (b), relationship between open probability and patch potential change (d) and open probability histograms (e). Data are mean ± SEM. ***P < 0.001 versus control and ### P < 0.001 versus pinacidil (e), one-way ANOVA with Newman-Keuls post hoc test
In preliminary experiments, pinacidil was selected as the most consistently effective KATP opener. Bath application of pinacidil 200 μM had a marked effect on channel activity highlighted by a 5.2-fold increase in channel NPo compared to control (Fig. 3c–e; P < 0.001). In our hands, there was no relationship between NPo and patch potential change as illustrated in Figs. 3d, 4c, 5c, 6c and 7b. There was no significant difference in sKATP unitary conductance with pinacidil (P > 0.05; see Table 1). The selective sKATP inhibitor HMR1098 10 μM had no effect on basal channel activity and NPo, P > 0.05, but effectively reduced pinacidil-induced sKATP openings and NPo (Fig. 3c–e) to basal levels (83 % reduction; 5.23 ± 1.20 versus 0.89 ± 0.18; P < 0.001). There was no significant difference in sKATP unitary conductance with HMR1098 (P > 0.05; see Table 1).
Representative sKATP recordings (a and b), the relationship between open probability and patch potential change (c) and open probability histograms (d and e). Data are mean ± SEM. ***P < 0.001 versus control (d), one-way ANOVA with Dunnett post hoc test; ***P < 0.001 versus control and ### P < 0.001 versus pinacidil (e), one-way ANOVA with Newman-Keuls post hoc test
Representative sKATP recordings (a and b), the relationship between open probability and patch potential change (c) and open probability histograms (d and e). Data are mean ± SEM. *P < 0.05 and ***P < 0.001 versus control (d), one-way ANOVA with Dunnett post hoc test; *P < 0.01 versus control, # P < 0.05 and ## P < 0.01 versus pinacidil (e), one-way ANOVA with Newman-Keuls post hoc test
Representative sKATP recordings (a and b), the relationship between open probability and patch potential change (c) and open probability histograms (d and e). Data are mean ± SEM. **P < 0.01 versus control (d), one-way ANOVA with Dunnett post hoc test; # P < 0.05 versus pinacidil (e), one-way ANOVA with Newman-Keuls post hoc test
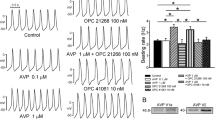
The effect of BNP and CNP on sKATP opening
Neither BNP nor CNP caused an appreciable increase in sKATP activity (Figs. 4a–e, 5a–e); in fact, bath application of either peptide resulted in a decrease in channel activity. BNP (≥10 nM) and CNP (≥0.01 nM) caused a significant decrease in sKATP NPo compared to control (Figs. 4a–e, 5a–e). For BNP and CNP, this decrease was concentration dependent (Figs. 4d, 5d). BNP at low concentrations (≤1 nM) had no effect on sKATP current and NPo (Fig. 4d). When either BNP (≥10 nM) or CNP (≥10 nM) was applied with pinacidil, the effects of the sKATP opener were completely abolished, highlighted by a marked reduction in channel NPo down to or below basal level. This significant effect was seen with BNP at all concentrations ≥10 nM [Fig. 4e: 2.28 ± 0.28 versus 0.50 ± 0.08 (10 nM), 0.49 ± 0.07 (100 nM), 1.03 ± 0.13 (1,000 nM); P < 0.001]. CNP at two out of three concentrations had similar effects [Fig. 5e: 1.61 ± 0.20 versus 0.83 ± 0.15 (10 nM), 0.58 ± 0.10 (100 nM); P < 0.05 and P < 0.01, respectively]. BNP (≤1 nM) did reduce pinacidil-stimulated sKATP currents but these effects did not reach significance (Fig. 4e; P > 0.05); furthermore, CNP applied at low concentrations (≤1 nM) was incapable of inhibiting pinacidil-stimulated sKATP currents (Fig. 5e; P > 0.05). These effects were not voltage dependent (Figs. 4c, 5c), and all treatments (see Table 1) had no significant effect on single channel unitary conductance compared to control.
The effect of C-ANF on sKATP activity
The NPR-C agonist C-ANF had interesting effects on sKATP activity. C-ANF (0.01 and 1 nM) had a negligible sKATP NPo; however, a 2.4-fold increase in NPo was seen with C-ANF 0.1 nM compared to control; however, this was not significant (Fig. 6d; P > 0.05). C-ANF (≥10 nM) augmented sKATP activity although a significant effect on sKATP NPo was only seen with C-ANF 100 nM (Fig. 6d; P < 0.01); nevertheless, C-ANF 10 and 1,000 nM caused an appreciable increase in sKATP activity (Fig. 6d). C-ANF 1 nM caused a significant blunting of pinacidil stimulated sKATP currents (2.54 ± 0.6 versus 1.22 ± 0.29 (1 nM); P < 0.05); however, this effect was not seen at all the other concentrations tested (Fig. 6e). The effects exhibited by C-ANF at all other concentrations were not statistically significant, although modest dampening of pinacidil stimulated sKATP activity is still evident at some concentrations (Fig. 6e; P > 0.05). There was no significant effect on single channel unitary conductance compared to control, and effects on NPo were not voltage dependent (P > 0.05; Fig. 6c and Table 1).
Cyclic GMP effect on sKATP activity
Unlike BNP and CNP, application of 8Br-cGMP (10 nM) did not cause a significant decrease in basal sKATP activity compared to control (P > 0.05). However, the compound attenuated sKATP responses to pinacidil when given simultaneously, causing a reduction in NPo (Fig. 7c). This 50 % relative reduction in NPo was significant (2.92 ± 0.60 versus 1.53 ± 0.32; P < 0.05). There was no appreciable effect on single channel conductance compared to control, and effects on NPo were not voltage dependent (P > 0.05; Fig. 7b and Table 1).
Discussion
Principal findings
Our data confirm the existence and expression of all KATP subunit genes and proteins in ventricular cardiomyocytes using RT-PCR and Western blotting and patch clamping, revealing a functional sKATP with biophysical and pharmacological properties consistent with that reported in the literature [46]. Our data also demonstrate a novel natriuretic peptide receptor mechanism of sKATP regulation in the cardiomyocyte under normoxic conditions. BNP (≤1 nM) had no effect on basal sKATP current but at high concentrations (≥10 nM) inhibited the ion channel, reducing NPo. BNP suppressed pinacidil-stimulated sKATP currents at all concentrations with the most marked effect seen at concentrations ≥10 nM. CNP (≥0.01 nM) suppressed basal sKATP openings, but only displayed this inhibitory action in presence of pinacidil at concentrations ≥10 nM. C-ANF (≥10 nM) had a marked stimulatory effect on basal sKATP; however, the effects of C-ANF at low concentrations (≤1 nM) are inconsistent. C-ANF had a negligible blunting effect on pinacidil-stimulated sKATP currents at all concentrations except at 1 nM. The action of BNP and CNP could potentially be associated with their ability to elevate intracellular concentrations of the second messenger cGMP, as it was demonstrated that the analog 8Br-cGMP was capable of dampening the pinacidil-activated sKATP current. As complete speculation, the stimulatory action of C-ANF at high concentrations (≥10 nM) could be associated with NPR-C mediated activation of PKC and subsequent sKATP opening [2, 24, 37].
Biomolecular, biophysical and pharmacological characterization of rat ventricular KATP
In this study, RT-PCR and Western blotting demonstrated the presence of Kir6.1, Kir6.2, SUR1 and SUR2 genes and proteins in rat left ventricular cardiomyocytes (Figs. 1, 2). Using immunofluorescence microscopy, Morrissey and colleagues confirmed the expression of all four KATP subunit proteins in rat ventricular cardiomyocytes, observing the co-localization of Kir6.2 and SUR2 subunits in the sarcolemma and transverse t-tubules [27]. Additionally, they found that Kir6.1 and SUR1 expression was particularly strong at the sarcolemmal surface [27]. Concerning the expression of SUR1 in our study, a strong band was detected at 70 kDa rather than the predicted 174 kDa. It is not known if the 70 kDa band was unmasked due to non-specific binding of the primary antibody, or if a SUR1 short form splice variant was detected. Splice variants of SUR1 have already been described in the heart [14, 18, 32]; however, their biological significance requires further elucidation.
The single channel unitary conductance of sKATP in symmetrical K+ (140 mM) conditions was between 50 and 60 pS (see Table 1). A sKATP was described in adult rat ventricular cardiomyocytes with a unitary conductance of 57.2 pS [46]. However, a considerable body of evidence has reported the unitary conductance of KATP in guinea pig [29], human [3], mouse [4], rabbit [29] and rat [48] ventricular cardiomyocytes to be between 70 and 80 pS under similar experimental conditions. This disparity in channel conductance reported in this study compared to that historically reported can be explained by the role of Kir6.X subunits in dictating KATP conductance [34]. Kir6.1 and Kir6.2 are highly homologous proteins that form a functional K+ channel when coupled to a SUR (1:1 tetrameric stoichiometry), with remarkably different unitary conductance. Under symmetrical K+ conditions, Kir6.1/SURX and Kir6.2/SURX have a divergent unitary conductance approximating 35 and 80 pS, respectively [34]. Chimeras of KATP have been described as exhibiting an intermediary unitary conductance between 55 and 65 pS [4, 12, 25, 42]. In two independent studies, the unitary conductance of KATP in cardiac cells isolated from mouse and rabbit purkinje fibers was demonstrated to be 57.1 pS [4] and 60.1 pS [25], respectively. Bao and colleagues proposed that the channel observed in their inside-out patch clamp experiments was a chimeric KATP [4]. Intriguingly following each attempted excision of the patch, channel activity completely disappeared, and according to Bao and co-workers, this could be indicative or a characteristic of a heteromeric KATP [4]. Morrissey and co-workers put forward the notion that a reassessment of the molecular composition of KATP in ventricular myocytes is needed, after elegantly showing strong sarcolemmal expression of Kir6.1 and SUR1 subunits by means of immunofluorescence [27]. In light of all the evidence, it is possible that a heteromeric KATP is present in the cardiac sarcolemma that presumably comprised two pore forming subunits of Kir6.1 and 6.2 coupled with SUR2A. This could explain why in our hands, a sarcolemmal K+ channel with features associated with KATP with a unitary conductance 50–60 pS was evident in our cell-attached patches.
In cell-attached patches, sKATP activity was markedly upregulated by the KATP opener pinacidil (Fig. 3c–e), an effect not seen with diazoxide (data not shown). This finding was not surprising because KATP with SUR1 (atrium) [14] and SUR2B (smooth muscle) [49] is highly sensitive to diazoxide, but not the SUR2A form (ventricle) [1]. Typically, pinacidil 200 μM increased sKATP NPo several fold above basal, an effect that was completely abolished by the selective inhibitor of the membrane form of KATP HMR1098 10 μM (Fig. 3d, e). HMR1098 did not reduce basal KATP openings (Fig. 3d, e). HMR1098 at concentrations ≥100 μM would be sufficient to reduce basal KATP opening [50]. These data provide pharmacological evidence that the K+ channel observed in cell-attached patches was sKATP.
Natriuretic peptide receptor modulation of rat ventricular KATP
Application of both BNP (≥10 nM) and CNP (≥0.01 nM) caused a marked and consistent depression of basal sKATP activity and NPo (Figs. 4, 5), contrary to our thinking that naturally occurring natriuretic peptides elicit/upregulate sKATP opening. The rationale behind our hypothesis that natriuretic peptides promote KATP opening was based on several studies in the setting of cardioprotection, showing that natriuretic peptide-induced limitation of infarct size involves KATP opening [5, 13, 47]. Thus, this study initially set out to investigate such a possibility by means of patch clamping, in particular the cell-attached configuration to maintain the intactness of intracellular signaling mechanisms, namely the natriuretic peptide receptor (NPR-A, NPR-B)/cGMP/protein kinase G (PKG) signaling cascade. The fascinating findings with both BNP and CNP on basal sKATP activity, together with their inhibitory effects on pinacidil evoked sKATP currents (Figs. 4, 5), appear to illustrate a novel mechanism of NPR-A and NPR-B regulation of sKATP in the heart. It is well known that natriuretic peptides play key roles in the cardiovascular adaptation to both acute and chronic pathological insult. The complexity of their fundamental roles as key mediators in multiple body systems, beyond the regulation of blood volume, is well documented, and it appears that the regulation of sKATP in the myocardium is an extension of this axis. Saegusa and colleagues demonstrated that ANP secretion from mechanically stretched mouse isolated atria was markedly enhanced in preparations taken from Kir6.2 deficient mice compared to wild type [38]. Speculatively, they suggested that sKATP could play a compensatory role in protecting the heart under pathological conditions. However, under physiological conditions, it could control stretch-induced ANP secretion via a negative feedback loop [38]. In a previous study, the sulphonylurea receptor ligand diazoxide, a KATP opener, was shown to inhibit stretch-induced ANP release in atrial cardiomyocytes [23], thus supporting the findings of Saegusa and colleagues [38].
BNP and CNP are agonists for different receptor-linked pGCs, namely NPR-A and NPR-B, respectively, and that both are capable of generating the second messenger cGMP. The fact that both BNP and ANP bind to the same receptor with the former having comparably lower affinity raises the possibility that the negative modulatory effects seen with BNP on KATP function can be recapitulated by ANP. Indeed Ropero and colleagues showed that ANP 1 nM dampened KATP activity in cell-attached patches from mouse pancreatic beta cells, illustrated by a 50 % reduction in NPo compared to no-treatment control [36]. The result obtained in Ropero’s study [36] is consistent with our findings that BNP and CNP are capable of inhibiting sKATP activity in rat ventricular cardiomyocytes.
The natriuretic peptides including BNP and CNP have a high affinity for the clearance receptor NPR-C [37]. Several sources of evidence suggest that some of the biological effects produced by natriuretic peptides are mediated through NPR-C, with evidence supporting a role for NPR-C in the hyperpolarization of vascular smooth muscle and endothelium [10, 44], and its role in CNP regulation of coronary blood flow and cardioprotection [22]. We sought to examine the role of NPR-C in natriuretic peptide regulation of sKATP using the NPR-C agonist C-ANF. Interestingly, C-ANF at concentrations ≥10 nM stimulated sKATP currents in our patch clamp experiments (Fig. 6). However, inhibition of pinacidil stimulated KATP currents was only observed with C-ANF 1 nM. These observations suggest that BNP and CNP do not elicit sKATP inhibition via NPR-C agonism.
cGMP as a modulator of KATP
The cGMP analog 8Br-cGMP 10 nM had no appreciable effect on sKATP openings under normoxic conditions, with no reduction in sKATP NPo compared to control, however, caused 50 % inhibition of pinacidil stimulated KATP openings (Fig. 7). Taking into consideration the results obtained with 8Br-cGMP, BNP (≥10 nM) and CNP (≥0.01 nM), the unexpected and novel inhibitory action of the natriuretic peptides on cardiac KATP activity may be at least partially associated with their ability to augment intracellular cGMP concentrations. However, a recent study by Chai and co-workers found that 8Br-cGMP 500 μM caused a threefold increase in KATP NPo in cell-attached patches from rabbit ventricular cardiomyocytes, although the representative recordings are somewhat unconvincing [9]. Furthermore, the concentration of cGMP used in this study is excessive, and massively in excess of intracellular cGMP concentration [16]. Interestingly, they found that the cell-permeable cGMP-phosphodiesterase inhibitor zaprinast (0.05–50 μM) increased KATP NPo in a concentration-dependent manner up to 12-fold above baseline, an effect that was completely blunted by addition of the PKG inhibitor KT5823 1 μM [9]. Their data show that cGMP-induced increase in sKATP activity in rabbit ventricular myocytes is in part PKG dependent. An earlier study by Han and colleagues examined the effect on NO on KATP activity in rabbit ventricular cardiomyocytes [20]. In cell-attached patches stimulated with pinacidil 50 μM, cumulative application of the NO-donors SNP or SNAP (0.1–1,000 μM) resulted in a concentration-dependent increase in KATP Po, an effect that was abolished by the KATP inhibitor glibenclamide 30 μM [20]. Furthermore, the potentiating effects of both NO-donors on pinacidil-induced KATP openings were abrogated by two structurally different PKG inhibitors Rp-8-Br-PET-cGMPS 10 μM and Rp-pCPT-cGMP 100 μM [20]. Similar findings were presented in a latter study, alluding to PKG activation as the key mechanism by which cGMP and NO-donors activate KATP [19] Fig. 8.
ANP/BNP and CNP bind cell surface receptors called NPR-A and NPR-B, respectively, which have an intracellular catalytic domain with guanylyl cyclase activity. NPR-A and NPR-B agonism leads to the generation of cGMP and activation of PKG. PKG phosphorylates serine/threonine residues in sKATP causing inhibition. The effect of NPR-A and NPR-B agonism on sKATP activity is mimicked by the cGMP analog 8Br-cGMP. C-ANF binds a distinct receptor devoid of a guanylyl cyclase domain called NPR-C and through a proposed Gαi-PLC-PIP2-DAG mechanism, activates PKC. PKC phosphorylates serine/threonine residues in sKATP leading to channel opening and an increase in sKATP activity. The PI3K/Akt/NOS and NO/sGC/cGMP signaling pathways have been proposed to interplay with the natriuretic pathway, augmenting natriuretic peptide generated pools of cGMP. These pools could potentially be responsible for facilitating sKATP inhibition
Taking all these findings into consideration, it appears that natriuretic peptides and NO have opposing effects on KATP activity cardiomyocytes, consistent with the differential effects observed with both autacoids despite generating the same second messenger [7, 8, 41]. Determining cGMP concentration following BNP and CNP administration in our pinacidil-activated preparation would give an interesting insight into the relationship between natriuretic peptide signaling and KATP activity. However, limitations remain using primary cultures of adult rat ventricular heart tissue that have prevented us from attempting such measurements relating to sufficient tissue, sensitivity to calcium during the isolation process and phenotypic stability [11].
Pathophysiological implications
The work described here has been undertaken in cardiomyocytes examined under standard electrophysiological conditions (normoxia). The technical limitations of the approach preclude the examination of natriuretic peptide effects on KATP under conditions of hypoxia or oxidative stress relevant to cardiac pathologies such as ischemia–reperfusion or ischemic cardiomyopathy. KATP is implicated in arrhythmia genesis [15], and mutations in genes coding for Kir6.2 (KCNJ11) and SUR2 (ABCC9) are linked to left ventricular hypertrophy and dilated cardiomyopathy in humans [30]. It will be relevant to attempt to model these in future studies. Although the concentrations of BNP and CNP employed in some experiments are many times higher than picomolar physiological plasma concentrations [35], they are very relevant to the interstitial concentrations in ventricular myocardium, especially in pathological states [35]. In conditions characterized by left ventricular dysfunction, such as chronic heart failure, release of stored BNP is observed (and there is some evidence to suggest CNP also), resulting in myocardial concentrations in the nanomolar region [35].
Conclusion
In conclusion, we have shown that BNP and CNP inhibit sKATP in rat ventricular cardiomyocytes and we believe this to be a novel NPR-A and NPR-B mechanism of KATP regulation in the heart, at least under physiological conditions. Examination of this regulatory mechanism in cardiomyocytes under conditions of oxygen deprivation and whether there are fundamental changes in natriuretic peptide regulation of KATP is important and warrants future investigation.
References
Ashcroft FM, Gribble FM (2000) Tissue-specific effects of sulfonylureas lessons from studies of cloned K-ATP channels. J Diabetes Complicat 14:192–196. doi:10.1016/s1056-8727(00)00081-7
Aziz Q, Thomas AM, Khambra T, Tinker A (2012) Regulation of the ATP-sensitive potassium channel subunit, Kir6.2, by a Ca2+ -dependent protein kinase C. J Biol Chem 287:6196–6207. doi:10.1074/jbc.M111.243923
Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J (1998) Reconstituted human cardiac K-ATP channels: functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res 83:1132–1143. doi:10.1161/01.RES.83.11.1132
Bao L, Kefaloyianni E, Lader J, Hong MY, Morley G, Fishman GI, Sobie EA, Coetzee WA (2011) Unique properties of the ATP-sensitive K+ channel in the mouse ventricular cardiac conduction system. Circ Arrhythm Electrophysiol 4:U235–U926. doi:10.1161/circep.111.964643
Burley DS, Baxter GF (2007) B-type natriuretic peptide at early reperfusion limits infarct size in the rat isolated heart. Basic Res Cardiol 102:529–541. doi:10.1007/s00395-007-0672-1
Burley DS, Hamid SA, Baxter GF (2007) Cardioprotective actions of peptide hormones in myocardial ischemia. Heart Fail Rev 12:279–291. doi:10.1007/s10741-007-9029-y
Castro LRV, Schittl J, Fischmeister R (2010) Feedback control through cGMP-dependent protein kinase contributes to differential regulation and compartmentation of cGMP in rat cardiac myocytes. Circ Res 107:1232–1240. doi:10.1161/circresaha.110.226712
Castro LRV, Verde I, Cooper DMF, Fischmeister R (2006) Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 113:2221–2228. doi:10.1161/circulationaha.105.599241
Chai YP, Zhang DM, Lin YF (2011) Activation of cGMP-dependent protein kinase stimulates cardiac ATP-sensitive potassium channels via a ROS/calmodulin/CaMKII signaling cascade. PLoS One 6(3):e18191. doi:10.1371/journal.pone.0018191
Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ (2003) Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA 100:1426–1431. doi:10.1073/pnas.0336365100
Chlopcikova S, Psotova J, Miketova P (2001) Neonatal rat cardiomyocytes–a model for the study of morphological, biochemical and electrophysiological characteristics of the heart. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 145:49–55. doi:10.5507/bp.2001.011
Cui Y, Giblin JP, Clapp LH, Tinker A (2001) A mechanism for ATP-sensitive potassium channel diversity: functional coassembly of two pore-forming subunits. Proc Natl Acad Sci USA 98:729–734. doi:10.1073/pnas.011370498
D’Souza SP, Yellon DM, Martin C, Schulz R, Heusch G, Onody A, Ferdinandy P, Baxter GF (2003) B-type natriuretic peptide limits infarct size in rat isolated hearts via K(ATP) channel opening. Am J Physiol Heart Circ Physiol 284:H1592–H1600. doi:10.1152/ajpheart.00902.2002
Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG (2008) Differential structure of atrial and ventricular K(ATP) atrial K(ATP) channels require SUR1. Circ Res 103:U1228–U1458. doi:10.1161/circresaha.108.178186
Flagg TP, Nichols CG (2005) Sarcolemmal K(ATP) channels: what do we really know? J Mol Cell Cardiol 39:61–70. doi:10.1016/j.yjmcc.2005.01.005
George WJ, Polson JB, O’Toole AG, Goldberg ND (1970) Elevation of guanosine 3′,5′-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci USA 66:398–403
Goetze JP, Gore A, Moller CH, Steinbruchel DA, Rehfeld JF, Nielsen LB (2004) Acute myocardial hypoxia increases BNP gene expression. FASEB J 18:1928–1930. doi:10.1096/fj.03-1336fje
Hambrock A, Preisig-Muller R, Russ U, Piehl A, Hanley PJ, Ray J, Daut J, Quast U, Derst C (2002) Four novel splice variants of sulfonylurea receptor 1. Am J Physiol Cell Physiol 283:C587–C598. doi:10.1152/ajpcell.00083.2002
Han J, Kim N, Joo H, Kim E, Earm YE (2002) ATP-sensitive K+ channel activation by nitric oxide and protein kinase G in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol 283:H1545–H1554. doi:10.1152/ajpheart.01052.2001
Han J, Kim N, Kim E, Ho WK, Earm YE (2001) Modulation of ATP-sensitive potassium channels by cGMP-dependent protein kinase in rabbit ventricular myocytes. J Biol Chem 276:22140–22147. doi:10.1074/jbc.M010103200
Haverkampf K, Benz I, Kohlhardt M (1995) Thermodynamically specific gating kinetics of cardiac mammalian K+(ATP) channels in a physiological environment near 37 degrees C. J Membr Biol 146:85–90. doi:10.1007/BF00232682
Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A (2004) Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation 110:1231–1235. doi:10.1161/01.cir.0000141802.29945.34
Jiao JH, Baumann P, Baron A, Roatti A, Pence RA, Baertschi AJ (2000) Sulfonylurea receptor ligands modulate stretch-induced ANF secretion in rat atrial myocyte culture. Am J Physiol Heart Circ Physiol 278:H2028–H2038
Light PE, Bladen C, Winkfein RJ, Walsh MP, French RJ (2000) Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proc Natl Acad Sci USA 97:9058–9063. doi:10.1073/pnas.160068997
Light PE, Cordeiro JM, French RJ (1999) Identification and properties of ATP-sensitive potassium channels in myocytes from rabbit Purkinje fibres. Cardiovasc Res 44:356–369. doi:10.1016/s0008-6363(99)00218-7
McLarnon JG, Hamman BN, Tibbits GF (1993) Temperature dependence of unitary properties of an ATP-dependent potassium channel in cardiac myocytes. Biophys J 65:2013–2020. doi:10.1016/S0006-3495(93)81243-8
Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA (2005) Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol 5:1. doi:10.1186/1472-6793-5-1
Nader L, Lahoud L, Chouery E, Aftimos G, Bois P, Fares NA (2010) B-type natriuretic peptide receptors in hypertrophied adult rat cardiomyocytes. Ann Cardiol Angeiol (Paris) 59:20–24. doi:10.1016/j.ancard.2009.09.009
Noma A (1983) ATP-regulated K+ channels in cardiac muscle. Nature 305:147–148. doi:10.1038/305147a0
Olson TM, Terzic A (2010) Human K-ATP channelopathies: diseases of metabolic homeostasis. Pflugers Arch 460:295–306. doi:10.1007/s00424-009-0771-y
Potter LR (2011) Natriuretic peptide metabolism, clearance and degradation. FEBS J 278:1808–1817. doi:10.1111/j.1742-4658.2011.08082.x
Pu J-L, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi N-Q (2008) Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive K-ATP activity. J Mol Cell Cardiol 44:188–200. doi:10.1016/j.yjmcc.2007.09.010
Rautureau Y, Gowers I, Wheeler-Jones CPD, Baxter GF (2010) C-type natriuretic peptide regulation of guanosine-3′,5′-cyclic monophosphate production in human endothelial cells. Auton Autacoid Pharmacol 30:185–192. doi:10.1111/j.1474-8673.2009.00449.x
Repunte VP, Nakamura H, Fujita A, Horio Y, Findlay I, Pott L, Kurachi Y (1999) Extracellular links in Kir subunits control the unitary conductance of SUR/Kir6.0 ion channels. EMBO J 18:3317–3324. doi:10.1093/emboj/18.12.3317
Richards AM, Troughton RW (2012) Use of Natriuretic Peptides to Guide and Monitor Heart Failure Therapy. Clin Chem 58:62–71. doi:10.1373/clinchem.2011.165704
Ropero AB, Soriano S, Tuduri E, Marroqui L, Tellez N, Gassner B, Juan-Pico P, Montanya E, Quesada I, Kuhn M, Nadal A (2010) The atrial natriuretic peptide and guanylyl cyclase-A system modulates pancreatic beta-cell function. Endocrinology 151:3665–3674. doi:10.1210/en.2010-0119
Rose RA, Giles WR (2008) Natriuretic peptide C receptor signalling in the heart and vasculature. J physiol 586:353–366. doi:10.1113/jphysiol.2007.144253
Saegusa N, Sato T, Saito T, Tamagawa M, Komuro I, Nakaya H (2005) Kir6.2-deficient mice are susceptible to stimulated ANP secretion: K-ATP channel acts as a negative feedback mechanism? Cardiovasc Res 67:60–68. doi:10.1016/j.cardiores.2005.03.011
Seino S, Iwanaga T, Nagashima K, Miki T (2000) Diverse roles of K-ATP channels learned from Kir6.2 genetically engineered mice. Diabetes 49:311–318. doi:10.2337/diabetes.49.3.311
Stenner E, Buiatti A, Barbati G, Merlo M, Sinagra G, Biasioli B (2012) Comparative evaluation of B-type natriuretic peptide and mid-regional pro-A-type natriuretic peptide changes from admission to discharge in prognosis of acute decompensated heart failure patients. Clin Lab 58:585–589
Su J, Scholz PM, Weiss HR (2005) Differential effects of cGMP produced by soluble and particulate guanylyl cyclase on mouse ventricular myocytes. Exp Biol Med 230:242–250
Teramoto N, Zhu HL, Shibata A, Aishima M, Walsh EJ, Nagao M, Cole WC (2009) ATP-sensitive K+ channels in pig urethral smooth muscle cells are heteromultimers of Kir6.1 and Kir6.2. Am J Physiol Renal Physiol 296:F107–F117. doi:10.1152/ajprenal.90440.2008
Uehlinger DE, Weidmann P, Gnadinger MP, Hasler L, Bachmann C, Shaw S, Hellmuller B, Lang RE (1986) Increase in circulating insulin induced by atrial natriuretic peptide in normal humans. J Cardiovasc Pharmacol 8:1122–1129. doi:10.1097/00005344-198611000-00005
Villar IC, Panayiotou CM, Sheraz A, Madhani M, Scotland RS, Nobles M, Kemp-Harper B, Ahluwalia A, Hobbs AJ (2007) Definitive role for natriuretic peptide receptor-C in mediating the vasorelaxant activity of C-type natriuretic peptide and endothelium-derived hyperpolarising factor. Cardiovasc Res 74:515–525. doi:10.1016/j.cardiores.2007.02.032
Westerlind A, Wahlander H, Berggren H, Lundberg P-A, Holmgren D (2008) Plasma levels of natriuretic peptide type B and A in children with heart disease with different types of cardiac load or systolic dysfunction. Clin Physiol Funct Imaging 28:277–284. doi:10.1111/j.1475-097X.2008.00805.x
Wu SN, Wu AZ, Sung RJ (2007) Identification of two types of ATP-sensitive K+ channels in rat ventricular myocytes. Life Sci 80:378–387. doi:10.1016/j.lfs.2006.09.042
Yang XM, Philipp S, Downey JM, Cohen MV (2006) Atrial natriuretic peptide administered just prior to reperfusion limits infarction in rabbit hearts. Basic Res Cardiol 101:311–318. doi:10.1007/s00395-006-0587-2
Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N (1997) The novel calcium sensitizer levosimendan activates the ATP-sensitive K+ channel in rat ventricular cells. J Pharmacol Exp Ther 283:375–383
Yokoshiki H, Sunagawa M, Seki T, Sperelakis N (1998) ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol Cell Physiol 274:C25–C37
Zhang HX, Akrouh A, Kurata HT, Remedi MS, Lawton JS, Nichols CG (2011) HMR 1098 is not an SUR isotype specific inhibitor of heterologous or sarcolemmal K ATP channels. J Mol Cell Cardiol 50:552–560. doi:10.1016/j.yjmcc.2010.12.011
Acknowledgments
This work was supported and funded by Cardiff University.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Burley, D.S., Cox, C.D., Zhang, J. et al. Natriuretic peptides modulate ATP-sensitive K+ channels in rat ventricular cardiomyocytes. Basic Res Cardiol 109, 402 (2014). https://doi.org/10.1007/s00395-014-0402-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-014-0402-4