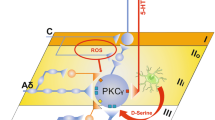
Abstract
Transient receptor potential receptors (TRP) on primary afferent neurons respond to noxious and/or thermal stimuli. TRPV1 receptors can be activated by noxious heat, acid, capsaicin and resiniferatoxin, leading to burning pain or itch mediated by discharges in C polymodal and Aδ mechano-heat nociceptors and in central neurons, including spinothalamic tract (STT) cells. Central nociceptive transmission involves both non-NMDA and NMDA receptors, and inhibitory interneurons as well as projection neurons contribute to the neural interactions. Behavioral consequences of intradermal injection of capsaicin include pain, as well as primary and secondary hyperalgesia and allodynia. Primary hyperalgesia depends on sensitization of peripheral nociceptors, whereas, secondary hyperalgesia and allodynia result from sensitization of central nociceptive neurons, such as STT cells. Central sensitization is associated with enhanced responses to excitatory amino acids and decreased responses to inhibitory amino acids. The mechanism of the increase in responses to excitatory amino acids includes phosphorylation of NR1 subunits of NMDA receptors and GluR1 subunits of AMPA receptors. Central sensitization depends on activation of several protein kinases and other enzymes, such as nitric oxide synthase. This process is regulated by protein phosphatases. Central sensitization can be regarded as a spinal cord form of long-term potentiation.



Similar content being viewed by others
References
Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC (1992) Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br J Pharmacol 107:544–552
Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824
Coderre TJ (1992) Contribution of protein kinase C to central sensitization and persistent pain following tissue injury. Neurosci Lett 140:181–184
Cortright DN, Szallasi A (2004) Biochemical pharmacology of the vanilloid receptor TRPV1: an update. Eur J Biochem 271:1814–1819
Dougherty PM, Willis WD (1991a) Modification of the responses of primate spinothalamic neurons to mechanical stimulation by excitatory amino acids and an N-methyl-d-aspartate antagonist. Brain Res 542:15–22
Dougherty PM, Willis WD (1991b) Enhancement of spinothalamic neuron responses to chemical and mechanical stimuli following combined micro-iontophoretic application of N-methyl-d-aspartic acid and substance P. Pain 47:85–93
Dougherty PM, Willis WD (1992) Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capsaicin-induced sensitization in the monkey. J Neurosci 12:883–894
Dougherty PM, Palecek J, Paleckova V, Sorkin LS, Willis WD (1992) The role of NMDA and non-NMDA excitatory amino acid receptors in the excitation of primate spinothalamic tract neurons by mechanical, chemical, thermal, and electrical stimuli. J Neurosci 12:3025–3041
Dougherty PM, Paleček J, Palečková V, Willis WD (1995) Infusion of substance P or neurokinin A by microdialysis alters responses of primate spinothalamic tract neurons to cutaneous stimuli and to iontophoretically released excitatory amino acids. Pain 61:411–425
Fang L, Wu J, Lin Q, Willis WD (2002) Calcium-calmodulin-dependent protein kinase II contributes to spinal cord central sensitization. J Neuroscience 22:4196–4204
Fang L, Wu J, Lin Q, Willis WD (2003a) Protein kinases regulate the phosphorylation of the GluR1 subunit of AMPA receptors of spinal cord in rats following noxious stimulation. Mol Brain Res 118:160–165
Fang L, Wu J, Zhang X, Lin Q, Willis WD (2003b) Increased phosphorylation of the GluR1 subunit of spinal cord α-amino-3-hydroxy-5-methyl-4-isoxazole proprionate receptor in rats following intradermal injection of capsaicin. Neuroscience 122:237–245
Fang L, Wu J, Zhang X, Lin Q, Willis WD (2005) Calcium/calmodulin dependent protein kinase II regulates the phosphorylation of cyclic AMP-responsive element-binding protein of spinal cord in rats following noxious stimulation. Neurosci Lett 374:1–4
Hardy JD, Wolff HG, Goodell H (1967) Pain sensations and reactions. Williams and Wilkins, 1952; reprinted by Hafner Publishing Co, New York
Holzer P (1991) Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev 43:143–201
Kennedy MB (2000) Signal-processing machines at the postsynaptic density. Science 290:750–754
LaMotte RH, Shain CN, Simone DA, Tsai EFP (1991) Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophyiol 66:190–211
LaMotte RH, Lundberg LER, Torebjörk HE (1992) Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol 448:749–764
Lin Q, Peng YB, Willis WD (1996a) Inhibition of primate spinothalamic tract neurons by spinal glycine and GABA is reduced during central sensitization. J Neurophysiol 76:1005–1014
Lin Q, Peng YB, Willis WD (1996b) Possible role of protein kinase C in the sensitization of primate spinothalamic tract neurons. J Neurosci 16:3026–3034
Lin Q, Peng YB, Willis WD (1997) Involvement of cGMP in nociceptive processing by and sensitization of spinothalamic neurons in primates. J Neurosci 17:3293–3302
Lin Q, Palecek J, Paleckova V, Peng YB, Wu J, Cui ML, Willis WD (1999a) Nitric oxide mediates the central sensitization of primate spinothalamic tract neurons. J Neurophysiol 81:1075–1085
Lin Q, Wu J, Peng YB, Cui ML, Willis WD (1999b) Inhibition of primate spinothalamic tract neurons by spinal glycine and GABA is modulated by guanosine 3′, 5′-cyclic monophosphate. J Neurophysiol 81:1095–1103
Lin Q, Wu J, Willis WD (2002) Effect of protein kinase A activation on the responses of primate spinothalamic tract neurons to mechanical stimuli. J Neurophysiol 88:214–221
Lisman JE, Zhabotinsky AM (2001) A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 31:191–201
Liu XG, Sandkühler J (1995) Long-term potentiation of C-fiber-evoked potentials in the rat dorsal horn is prevented by spinal N-methyl-d-aspartic acid receptor blockage. Neurosci Lett 191:43–46
Liu XG, Sandkühler J (1997) Characterization of long-term potentiation of C-fiber-evoked potentials in spinal cord dorsal horn of adult rat: essential role of NK1 and NK2 receptors. J Neurophysiol 78:1973–1982
Liu XG, Sandkühler J (1998) Activation of spinal N-methyl-d-aspartate or neurokinin receptors induced long-term potentiation of spinal C-fibre-evoked potentials. Neuroscience 86:1209–1216
Malenka RC, Nicoll RA (1999) Long-term potentiation- a decade of progress? Science 285:1870–1874
Montell C, Jones K, Hafen E, Rubin G (1985) Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 230:1040–1043
Ochoa J, Torebjörk E (1989) Sensations evoked by intraneural microstimulation of C nociceptor fibres in human skin nerves. J Physiol 415:583–599
Palecek J, Paleckova V, Dougherty PM, Willis WD (1994) The effect of phorbol esters on the responses of primate spinothalamic neurons to mechanical and thermal stimuli. J Neurophysiol 71:529–537
Patapoutian A, Peier AM, Story GM, Viswanath V (2003) ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci 4:529–539
Randic M, Jiang MC, Cerne R (1993) Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci 13:5228–5241
Rygh LJ, Svenden F, Hole A, Tjølsen A (1999) Natural noxious stimulation can induce long-term increase of spinal nociceptive responses. Pain 82:305–310
Sandkühler J (2000) Learning and memory in pain pathways. Pain 88:113–118
Sandkühler J, Liu X (1998) Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. Eur J Neurosci 10:2476–2480
Sang CN, Gracely RH, Max MB, Bennett GJ (1996) Capsaicin-evoked mechanical allodynia and hyperalgesia cross nerve territories: evidence for a central mechanism. Anesthesiology 85:491–496
Schmelz M, Schmidt R, Forster C, Ringkamp M, Torebjörk HE (1994) Sensitization of insensitive branches of C nociceptors in human skin. J Physiol 480:389–394
Sluka KA (1997) Activation of the cAMP transduction cascade contributes to the mechanical hyperalgesia and allodynia induced by intradermal injection of capsaicin. Br J Pharmacol 122:1165–1173
Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD (1997) Inhibitors of G-proteins and protein kinases reduce the sensitization to mechanical stimulation and the desensitization to heat of spinothalamic tract neurons induced by intradermal injection of capsaicin in the primate. Exp Brain Res 115:15–24
Sorkin LS, McAdoo DJ (1993) Amino acids and serotonin are released into the lumbar spinal cord of the anesthetized cat following intradermal capsaicin injection. Brain Res 607:89–98
Sorkin LS, Westlund KN, Sluka KA, Dougherty PM, Willis WD (1992) Neural changes in acute arthritis in monkeys. IV. Time-course of amino acid release into the lumbar dorsal horn. Brain Res Rev 17:39–50
Sun RQ, Lawand NB, Lin Q, Willis WD (2004a) Role of calcitonin gene-related peptide in the sensitization of dorsal horn neurons to mechanical stimulation after intradermal injection of capsaicin. J Neurophysiol 92:320–326
Sun RQ, Tu Y, Lawand NB, Yan JY, Lin Q, Willis WD (2004b) Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol 92:2859–2866
Sun RQ, Yan J, Willis WD (2006) Activation of protein kinase B/Akt signaling pathway contributes to mechanical hypersensitivity induced by capsaicin. Pain 120:86–96
Svendsen F, Tjølsen A, Hole K (1998) AMPA and NMDA receptor-dependent spinal LTP after nociceptive tetanic stimulation. Neuroreport 9:1185–1190
Szallasi A, Blumberg PM (1999) Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev 51:159–211
Szolcsányi J (1993) Actions of capsaicin on sensory receptors. In: Wood JN (ed) Capsaicin in the study of pain. Academic Press, London, pp 1–26
Szolcsányi J, Anton F, Reeh PW, Handwerker HO (1988) Selective excitation by capsaicin of mechano-heat sensitive nociceptors in rat skin. Brain Res 446:262–268
Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nillus B (2004) The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430:748–754
Willis WD (1999) Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res 124:395–421
Willis WD (2002) Long-term potentiation in spinothalamic neurons. Brain Res Reviews 40:202–214
Willis WD (2006) The nociceptive membrane: historical overview. Current Topics Membr 57:74–111 Elsevier Inc
Willis WD (2007) The somatosensory system, with emphasis on structures important for pain. Brain Res Reviews 55:297–313
Wu J, Lin Q, McAdoo DJ, Willis WD (1998) Nitric oxide contributes to central sensitization following intradermal injection of capsaicin. Neuroreport 9:589–592
Wu J, Fang L, Lin Q, Willis WD (2001) Nitric oxide synthase in spinal central sensitization following intradermal injection of capsaicin. Pain 94:47–58
Yan JY, Sun RQ, Hughes MG, McAdoo DJ, Willis WD (2006) Intradermal injection of capsaicin induces acute substance P release from rat spinal cord dorsal horn. Neurosci Lett 410:183–186
Zhang X, Wu J, Fang L, Willis WD (2003) The effects of protein phosphatase inhibitors on nociceptive behavioral responses of rats following intradermal injection of capsaicin. Pain 106:443–451
Zhang X, Wu J, Lei Y, Fang L, Willis WD (2005) Protein phosphatase modulates the phosphorylation of spinal cord NMDA receptors in rats following intradermal injection of capsaicin. Mol Brain Res 138:264–272
Zhang X, Wu J, Fang L, Willis WD (2006a) The effects of protein phosphatase inhibitors on the duration of central sensitization of rat dorsal horn neurons following injection of capsaicin. Mol Pain 2:23
Zhang X, Wu J, Lei Y, Fang L, Willis WD (2006b) Protein phosphatase 2A regulates central sensitization in the spinal cord of rats following intradermal injection of capsaicin. Mol Pain 2:9–20
Zou X, Lin Q, Willis WD (2000) Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neuroscience 20:6989–6997
Zou X, Lin Q, Willis WD (2001) NMDA or non-NMDA receptor antagonists attenuate increased FOS expression in spinal dorsal horn GABAergic neurons after intradermal injection of capsaicin in rats. Neuroscience 106:171–182
Zou X, Lin Q, Willis WD (2002) Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience 115:775–786
Zou X, Lin Q, Willis WD (2004) Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Res 1020:95–105
Acknowledgments
Experiments done in the author’s laboratory were supported by grants NS09743 and NS11255. The author thanks Kelli Gondesen for technical assistance and Griselda Gonzales for assistance with the illustrations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willis, W.D. The role of TRPV1 receptors in pain evoked by noxious thermal and chemical stimuli. Exp Brain Res 196, 5–11 (2009). https://doi.org/10.1007/s00221-009-1760-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1760-2