Abstract
Rationale
Activation of one or more of the serotonin (5-HT) receptors may play a role in mediating the antidepressant effects of SSRIs.
Objective
The present studies were conducted to evaluate the effects of the novel 5-HT2C receptor agonist WAY-163909 in animal models of antidepressant activity (forced swim test (FST), resident–intruder, olfactory bulbectomy (BULB)), in a schedule-induced polydipsia (SIP) model of obsessive–compulsive disorder and in a model for evaluating sexual dysfunction.
Results
WAY-163909 (10 mg/kg, i.p. or s.c.) decreased immobility time in Wistar–Kyoto rats in the FST, effects that were reversed by the 5-HT2C/2B receptor antagonist SB 206553. Moreover, in Sprague-Dawley rats, the profile of WAY-163909 (decreased immobility, increased swimming) in the FST was comparable to the effects of SSRIs. Acute treatment with WAY-163909 (0.33 mg/kg, s.c.) decreased rodent aggression at doses lower than those required for decreasing total behavior. Administration of WAY-163909 (3 mg/kg, i.p.) for 5 or 21 days decreased the BULB-induced hyperactivity in rats. Additionally, acute administration of WAY-163909 (3 mg/kg, i.p.) decreased adjunctive drinking in a SIP model. The effects of WAY-163909 were reversed by the 5-HT2C/2B receptor antagonist SB 206553 and the selective 5-HT2C receptor antagonist SB 242084. Chronic administration of WAY-163909 produced deficits in sexual function at doses higher (10 mg/kg, i.p.) than those required for antidepressant-like effects in the BULB model.
Conclusions
Taken together, these results demonstrate that the novel 5-HT2C receptor agonist WAY-163909 produces rapid onset antidepressant-like effects in animal models and may be a novel treatment for depression.
Similar content being viewed by others
Introduction
Selective serotonin reuptake inhibitors (SSRIs) increase levels of synaptic serotonin, which acts at the different serotonin (5-HT) receptor subtypes (as many as 14 different receptors). The antidepressant effects of SSRIs are likely mediated by one or more of these receptors, but it is unlikely that all 14 play a critical role. Additionally, the undesired side effects of SSRIs are likely mediated by activation of one or more of these receptors, which may be distinct from those that mediate antidepressant action. Moreover, due to feedback regulation by 5-HT1A, 5-HT1B, and 5-HT1D receptors, chronic administration of SSRIs is necessary to sustain increases in serotonin levels, consistent with the 2–3 week delay required for antidepressants to become effective (Dawson et al. 2000; Beyer et al. 2002). Therefore, by specifically targeting a postsynaptic serotonin receptor, it may be possible to improve onset of action.
One candidate 5-HT receptor supported by preclinical literature, as early as the 1990s, for mediating the antidepressant-like effects of SSRIs is the 5-HT2C receptor. 5-HT2C receptor agonists show antidepressant-like effects in multiple animal models (both acute and chronic) of depression. For example, 5-HT2C receptor agonists decrease immobility time and increase swimming time in the FST in rats in a manner comparable to SSRIs (Cryan and Lucki 2000). The effects of the 5-HT2C receptor agonists and SSRIs in the rat forced swim test are antagonized by the 5-HT2C antagonists (Cryan and Lucki 2000) consistent with the role for 5-HT2C receptors in mediating antidepressant-like effects of 5-HT2C receptor agonists and SSRIs. Additional studies with 5-HT2C receptor agonists have shown that they are effective in multiple models of antidepressant action including the DRL-72 s (differential reinforcement of low rate) model, the resident–intruder model (Mitchell and Redfern 2000, 2005), the BULB model, and the chronic mild stress model (Moreau et al. 1996; Martin et al. 1998). Several studies are consistent with the idea that 5-HT2C receptor agonists will have rapid onset antidepressant effects. In the chronic mild stress model and in a BULB model, antidepressants typically require 2–3 weeks of administration to show effectiveness. 5-HT2C receptor agonists are effective by day 3 in the chronic mild stress model and are effective after short-term treatment in the BULB model, consistent with rapid onset antidepressant-like effects (Moreau et al. 1993, 1996; Martin et al. 1998).
WAY-163909 is a novel compound that binds with high affinity (Ki = 11 nM) and functions as a full agonist (EC50 = 8 nM; Emax = 90% relative to 5-HT) at the 5-HT2C receptor (Dunlop et al. 2005). WAY-163909 shows selectivity for 5-HT2C receptors relative to 5-HT2A (20-fold) and 5-HT2B (46-fold) and shows no agonist activity at the 5-HT2A receptor and partial agonism at the 5-HT2B receptor (Emax = 40%; relative to 5-HT). Relative to other compounds reported in the literature including Org 37684 (Leyson and Kelder 1998), Ro 60-0175 (Martin et al. 1998), WAY-161503 (Rosenzweig-Lipson et al. 2006; Welmaker et al. 2000), PNU-22394 (McCall et al. 2001), VER-3323 (Bickerdike et al. 2002), YM348 (Kimura et al. 2004), and WAY-629 (Sabb et al. 2004), WAY-163909 appears to be more selective with respect to 5-HT2C binding and functional selectivity. These enhanced properties would suggest that WAY-163909 will have a low propensity for 5-HT2A/B receptor mediated side effects. WAY-163909 is orally active, highly brain penetrant, and has approximately a 4-h half-life (Dunlop et al. 2006). Moreover, WAY-163909 decreases food intake and body weight in animal models of obesity (Dunlop et al. 2005) and is effective in multiple animal models of schizophrenia (Marquis et al. 2007).
The present studies were conducted to evaluate the effects of the novel 5-HT2C agonist, WAY-163909, in multiple animal models of antidepressant activity (FST, resident–intruder, BULB). These models were chosen based on their sensitivity to a range of antidepressant mechanisms. Both the forced swim test and the resident–intruder model are sensitive to the effects of antidepressants after acute administration. Wistar rats were chosen for the resident–intruder studies as the baseline levels of aggression are amenable to either decreases or increases by antidepressants after acute or chronic administration, respectively (Mitchell and Redfern 2005). The BULB model requires 14–21 days of administration for current clinically effective antidepressants to be effective, providing a window for a compound to demonstrate rapid onset. In addition, the effects of WAY-163909 were also evaluated on SIP, a model suggested to predict efficacy for obsessive–compulsive disorder (OCD) as well as to predict rapid onset antidepressant-like effects (Woods et al. 1993; Hogg and Dalvi 2004). As SSRIs produce marked effects on sexual function, WAY-163909 was also evaluated in a sexual dysfunction model to determine the potential for this side effect associated with antidepressants. Overall, WAY-163909 produces antidepressant-like effects in all animal models of antidepressant-like activity with a rapid onset profile and limited sexual side effect liability.
Materials and methods
Subjects
Male Sprague-Dawley (SD) rats weighing 175–250 g upon arrival from Charles River Laboratories were used for FST, BULB, SIP, and sexual dysfunction studies. Ovariectomized female Long–Evans rats (Charles River Laboratories) were individually housed and used as stimuli in the sexual dysfunction model. Male Wistar–Kyoto (WKY) rats weighing 190–225 g upon arrival from Charles River were also used in the forced swim test. Male Wistar rats from Charles River UK were used as residents in the resident–intruder model, and male Wistar rats from Bath University stock weighing 300–350 g at the start of the study were used as intruders.
Assurances
All experimental procedures conducted at Wyeth were carried out in accordance with protocols approved by the Wyeth Institutional Animal Care and Use Committee, and all animals were housed in an AAALAC-accredited facility that was maintained on a 12-h light/dark cycle (lights on at 0600 hours). The forced swimming test studies conducted at the University of Pennsylvania were carried out in accordance with protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee. Studies of resident–intruder aggression performed at the University of Bath were conducted under a project license held under the Animals (Scientific Procedures) Act 1986 (UK) and in accordance with the principles of laboratory animal care (NIH publication No. 85–23, revised 1996).
Forced swim test
The SD or WKY rats were allowed to acclimate for at least 3 days before testing and then were handled daily for 3–5 days before testing. The forced swim procedure used was similar to that of Porsolt et al. (1977) and Detke et al. (1995). Two swim sessions were conducted between 12:00 noon and 5:00 p.m. across two consecutive days. In the first session (pretest), rats were placed individually into a glass cylinder (46 cm tall × 20 cm diameter) filled with water to a depth of 30 cm (approximately 25 degrees centigrade) for 15 min. The pretest was followed 24 h later by a 5 min test session. After each session, the rat was dried with a paper towel and returned to its home cage. Tank water was changed after each rat on both pretest and test days. WAY-163909 (1–10 mg/kg, SD; 3–17 mg/kg, WKY) or saline was administered i.p., 15 min after the pretest swim session, as well as 5 and 1 h before the test session. Housed pairs of animals received the same treatment. For antagonism experiments in WKY rats, SB 206553 (20 mg/kg, i.p.) and WAY-163909 (17 mg/kg, i.p.) were each injected at all three time points. Dose, pretreatment times, and route of administration for SB 206553 were based on literature (Cryan and Lucki 2000).
In the SD rats, manual scoring of the behavioral patterns was carried out by an investigator blind to the experimental conditions of the animals being scored. A time sampling technique was used whereby the predominant behavior in each 5-s period of the 300-s test was recorded. Climbing behavior consisted of upward-directed movements of the forepaws along the side of the swim chamber. Swimming behavior was defined as movement (usually horizontal) throughout the swim chamber, which usually included crossing into another quadrant. Immobility was assigned when no additional activity was observed other than that required to keep the rat’s head above water. Fisher’s protected least significant difference (PLSD) tests (p < 0.05) were used to compare differences between individual doses and the saline control.
In the WKY rats, immobility was measured using the mobility detection parameter of the Ethovision videotracking software (Noldus). Immobility was defined by a threshold setting of 14%. Immobility times were used for statistical analysis.
Resident–intruder
The procedures used were those described by Mitchell and Redfern (for full details see Mitchell and Redfern 2005). All resident and intruder rats were housed in closed social groups of four rats per group for at least 5 weeks immediately before and throughout (intruder groups only) each experiment.
Social encounters were performed at weekly intervals and arranged to ensure that each of the resident rats encountered a different intruder rat from the corresponding intruder group over four test sessions. Over these sessions, each resident rat was treated with saline and WAY-163909 (0.33, 1.0 and 3.0 mg/kg s.c.) administered in a random design. Intruder rats remained untreated. All social encounter studies were performed between 9 a.m. and 2 p.m. with the experimental room under low-intensity red-light illumination (2–6 lx). Resident animals were separated 3 days before each test day and housed individually. Thirty minutes before the social encounter, the resident rats were treated with WAY-163909 and then allowed to habituate to the recording cabinet (described in Mitchell and Redfern 1992) after which an unfamiliar intruder rat was introduced into the resident rat’s home cage and the ensuing social encounter recorded on to video tape for 10 min. During the experiment and subsequent behavioral analysis, the operator was unaware of each treatment administered to the resident rats. After each social encounter, the resident and intruder rats were returned to their respective group cages.
Ethological analysis of the resident rat’s behavior was always performed blind to the treatment administered to each resident rat. From these records the frequency of each behavior/posture exhibited during each social encounter was calculated, grouped according to the motivational category in which that behavior occurs, and the total score for each category was expressed as a percentage of the total number of behaviors observed for that animal. These categories have been described previously (Mitchell and Redfern 2005) and include exploration, investigation, sexual, aggression, flight-submit, flight-escape, and maintenance behaviors. Experience has shown that data arising from the ethological scoring method are generally positively skewed. All behavioral data were thus subjected to square root transformation before statistical analysis. All statistical procedures were performed using SuperANOVA™ (Abacus Concepts, Macintosh). Data were grouped according to treatment and the mean and standard error of the mean (SEM) for both the percentage values of each motivational category and the total number of behaviors/postures calculated. The head/body shake data were also examined in detail separately. Data were subjected to repeated measures analysis of variance (ANOVA) across treatment for each category of behavior. Where significant main effects of treatment were identified then significant differences from vehicle control treatment were determined using the Bonferroni/Dunn (control) post hoc t test. In addition, the dose estimated to inhibit a particular behavior by 50% (inhibitory dose 50%, ID50) was calculated by the least squares method together with the appropriate 95% confidence limits.
Olfactory bulbectomy
Rats were group-housed until surgery and pair-housed with another rat given the same surgical treatment throughout the remainder of the experiment.
A bilateral BULB or sham surgery was performed on all rats. Animals were anesthetized with pentobarbital (75 mg/kg). The surgical procedure consisted of drilling two burr holes (2 mm diameter), 8.0 mm from bregma, ±2.0 mm from the midline. The olfactory bulbs were completely removed by aspiration via vacuum pump. Sham-operated animals were treated identically except that their olfactory bulbs were not removed. Hemostatic sponge was placed in the holes to prevent blood loss, and the wounds were closed by using wound clips. All surgical manipulations were performed in accordance with Wyeth Institutional Animal Care and Use Committee guidelines and approvals.
All animals were weighed and handled daily for 14 days after surgery before receiving any drug or saline injections. WAY-163909 or saline was administered s.c. for either 5 or 21 days. On the last day of drug administration 4-h after dosing, animals were placed in the open field apparatus for a 5-min locomotor activity test. The open field apparatus was a 90 × 90 cm opaque Plexiglas box with 30 cm high sides. A camera connected to a computer and software analysis program (Noldus Ethovision) was suspended over the open field to track the location of the rat during the test. For scoring, the total distance (cm) moved was recorded by the software program. For each treatment group, the average distance moved was calculated and used for statistical analysis.
At the end of the study, the BULBs were evaluated histologically. Only subjects with complete bilateral BULBs and no other damage were included in the data analysis.
Schedule-induced polydipsia
Body weights were reduced gradually to 85% of baseline weights by restricting post-session feeding.
Rats were placed in an operant conditioning chamber (MED Associates) equipped with a pellet dispenser, a food receptacle, a houselight, and a water bottle with 100 ml of water. Houselights were turned on in the chamber, and a food pellet (Bioserve, 45 mg) was delivered on a fixed-time 60 s schedule, such that one pellet was delivered each minute during a 2-h experimental session. At the end of the session, water intake was measured.
Experiments to control for schedule and baseline water intake were conducted by placing 120 pellets in the food receptacle at the start of a 2-h experimental session. Houselights were turned on, but no additional food pellets were delivered. At the end of the session, water intake was measured.
WAY-163909 (1–5.6 mg/kg) was administered i.p. in a volume of 1 ml/kg immediately before the 2-h experimental session. For the 1st set of antagonism studies, SB 206553 (10 mg/kg, i.p.) was administered 1 h before the administration of 5.6 mg/kg WAY-163909. Due to greater sensitivity of WAY-163909 in another group of rats, in additional antagonism studies, SB 242084 (1 mg/kg, i.p.) or SB 215505 (3 mg/kg, p.o.) were administered 1 h before the administration of 3 mg/kg WAY-163909. Doses, pretreatment times, and route of administration for antagonists were determined based on literature (SB 206553—Cryan and Lucki 2000; SB 242084—Kennett et al. 1997; SB 215505—Kennett et al. 1998) or previous studies with WAY-163909 in combination with these antagonists (Dunlop et al. 2005; Marquis et al. 2007).
Sexual dysfunction
Intact male SD rats were allowed to habituate for 1 week before sexual experience or behavioral testing. Ovariectomized female Long–Evans rats were individually housed and brought into behavioral estrus weekly by subcutaneous injection of 25 μg 17-β estradiol benzoate in 0.1 ml corn oil 48 h before testing, followed by subcutaneous administration of 2.5 mg progesterone in 0.1 ml corn oil 6 h before testing.
Individual male rats were placed into the home cage of a sexually experienced and receptive female for overnight mating sessions. This was a Plexiglas cage measuring 25.4 × 30.5 cm, with food and water ad libitum. Mating pairs were randomly assigned. The interaction between the male and female rats was observed for the first hour. Only males that were observed for intromission within the first hour of paired mating sessions were included in behavioral studies. After overnight mating sessions, male and female rats were separated from each other and placed back into same-sex colony rooms where they were group-housed until the time of behavioral testing.
After sexual experience (as described above), male subjects (n = 6–8) were administered either 3 or 10 mg/kg/day WAY-163909 for 14 days. Noncontact penile erections were evaluated in 30 min test sessions in the presence of sexually receptive female rats as described below.
Sexually receptive females were brought into the testing room 30 min before the start of behavioral testing to provide female visual, olfactory, and auditory stimuli for males. The testing arena for observing noncontact penile erections in male rats consisted of an empty rat size Plexiglas cage (27.9 × 25.4 cm) containing no bedding, with an aerated plastic lid and a total ceiling height of 30.5 cm. Males were observed for noncontact penile erections in individual test cages. A penile erection consisted of observation of the male rat in a hunched position grasping the penis with the forepaws and followed by a series of pelvic thrusts. The number of penile erections was quantified over a 30-min observation session.
Data are expressed as mean number of noncontact penile erections per treatment group, calculated as percent of vehicle control ± SEM.
Compound preparation
WAY-163909 ((7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]-diazepino[6,7,1hi]indole) was synthesized at Wyeth Research. WAY-163909 was dissolved in saline and administered by the specified route in a dose volume of 1–2 ml/kg. SB 206553 was dissolved in 2% Tween/0.5% methylcellulose, SB 242084 was dissolved in 10% Tween/0.5% methylcellulose, and SB 215505 was dissolved in 5% dextrose and were administered i.p. in a dose volume of 1 ml/kg. All compounds were prepared fresh daily, and doses are expressed on the basis of active moiety.
Statistical analysis
Unless otherwise noted [forced swim test (FST)—SD rats; Resident–Intruder] all data were subjected to one-way ANOVA with a least significant difference (LSD) post hoc test comparing treatment groups to vehicle as appropriate. Statistical significance was achieved when p < 0.05 compared to the vehicle condition.
Results
FST—WKY rats and SD rats
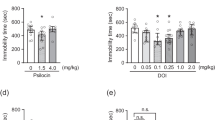
In WKY rats, WAY-163909 significantly reduced immobility time in the rat FST [F(3,27) = 3.24, p < 0.05]. Post hoc comparisons revealed that both 10 and 17 mg/kg i.p. significantly reduced immobility in the rat forced swim test (p values <0.05; Fig. 1a). The 5-HT2C/2B receptor antagonist SB 206553 (20 mg/kg, i.p.) was administered as a cotreatment with WAY-163909 (17 mg/kg, i.p.). As expected, vehicle + WAY-163909 produced a significant decrease in immobility time, whereas SB 206553 + vehicle had no effect on immobility time. SB 206553 fully blocked the decrease in immobility time produced by WAY-163909 (Fig. 1b).
(a) Effect of WAY-163909 on the immobility time in the rat forced swim test in WKY rats. (b) Effect of SB-206553 on the reduction in immobility produced by WAY-163909. Values represent mean immobility time ± 1 SEM. Asterisks indicate values differ from vehicle treatment (p < 0.05). Number sign indicates values differ from WAY-163909 (p < 0.05). N = 12 per treatment group
Similarly, in SD rats, WAY-163909 decreased immobility at 10 mg/kg when compared with rats treated with saline (p < 0.05; Fig. 2). Analysis of active behaviors showed that 10 mg/kg, i.p. WAY-163909 produced a corresponding increase in swimming behavior (p < 0.001) but produced no significant effect on climbing behavior.
Resident–intruder
Repeated measures ANOVA across treatments revealed significant main effects of acute WAY-163909 treatment (Table 1) on investigatory, aggressive, flight escape, maintenance and sexual behavior, and total behaviors observed [all Fs(3,18) ≥ 7.3, p ≤ 0.003 in all cases]. In contrast, no main effects of treatment were observed on exploration, flight submit or head/body shake behavior. Compared to the behavioral profile expressed after drug–vehicle treatment, post hoc analysis revealed that WAY-163909 significantly reduced aggressive behavior at all three doses (p values <0.05 in all cases) with an ID50 value of 0.33 mg/kg, s.c. (95% CI: 0.055–0.719 mg/kg, s.c.). Although all three doses also decreased the number of total behaviors, the ID50 value was approximately threefold higher [i.e., 0.967 mg/kg, s.c. (95% CI: 0.755–1.234 mg/kg, s.c.)]. Other behaviors such as investigation, sexual behavior, and flight escape were also significantly affected at 1 or 3 mg/kg (Table 1).
Olfactory bulbectomy
Consistent with literature findings, BULB increased distance traveled compared to the SHAM condition (p < 0.0001; Fig. 3 left). After chronic treatment with WAY-163909 (1–3 mg/kg, i.p. 21 days), the effect of BULB on distance traveled was dose-dependently attenuated by WAY-163909 [F(5,38) = 10.308, p < 0.0001; Fig. 3 left]. The increase in activity was partially attenuated by 1 mg/kg of WAY-163909 (p = 0.039 vs BULB-saline and p = 0.0051 vs SHAM-saline) and fully attenuated after 3 mg/kg of WAY-163909 (p < 0.0001 vs BULB-saline and n.s. vs SHAM-saline). WAY-163909 did not affect activity levels of SHAM rats treated with WAY-163909, consistent with a specific effect of WAY-163909 on BULB induced hyperactivity.
Effects of WAY-163909 on distance traveled in the olfactory bulbectomy model following 21-day (left) or 5-day (right) administration. Values represent distance traveled (cm) ± 1 SEM. Asterisks indicate values differ from SHAM-saline treatment (p < 0.05) and number signs indicate values differ from BULB-saline treatment. N = 5–8 per treatment group
Similarly, after a short-term treatment with WAY-163909 (3 mg/kg, 5 days), the BULB-induced increases in distance traveled were attenuated by WAY-163909 [F(3,29) = 11.042, p < 0.0001; Fig. 3 right]. Post hoc analysis revealed that distance traveled was significantly increased in BULB rats treated with vehicle compared to SHAM rats treated with vehicle (p < 0.0001). The increase in activity was fully attenuated after 3 mg/kg of WAY-163909 (p < 0.018 vs BULB-saline and n.s. vs SHAM-saline). WAY-163909 did not affect activity levels of SHAM rats treated with WAY-163909, consistent with a specific effect of WAY-163909 on BULB induced hyperactivity.
Schedule-induced polydipsia
After administration of saline, rats drank 61.7 ± 3.2 ml of water during the 2 h experimental session during which a food pellet was delivered once per minute. WAY-163909 (1–5.6 mg/kg, i.p.) produced dose-dependent decreases in excessive water intake in the SIP procedure [F(4,27) = 11.8; p < 0.001; Fig. 4a). Post hoc tests revealed that 3 mg/kg (−41%; p = 0.0006) and 5.6 mg/kg (−73%; p < 0.0001) produced significant decreases in excessive water intake. The ED50 value for WAY-163909 was 3.9 mg/kg, i.p. (95% CI: 2.7–5.5 mg/kg, i.p.). In additional studies, rats were given 120 pellets at the start of the 2-h session and were given free access to water. Under these conditions, after administration of saline, rats drank 9.4 ± 2.0 ml of water. WAY-163909 (3–5.6 mg/kg, i.p.) had no effect on water intake under this condition.
(a) Effects of WAY-163909 on water intake in the SIP model. (b) Effects of the 5-HT2C/2B antagonist SB 206553 on WAY-163909 decreases in SIP. (c) Effects of the 5-HT2C selective antagonist SB 242084 or the 5-HT2B selective antagonist SB 2155505 on WAY-163909 decreases in SIP. Values represent the mean of the percentage of baseline values ± 1 SEM. Asterisks indicate values differ from saline treatment p < 0.05) and number signs indicate values differ from 5.6 mg/kg WAY-163909 alone. nt, not tested. N=5–6 per treatment group
Pretreatment with the 5-HT2C/2B antagonist SB 206553 (10 mg/kg, i.p.) blocked the decrease in adjunctive drinking produced by 5.6 mg/kg WAY-163909 (Fig. 4b; p < 0.0006 vs 5.6 mg/kg WAY-163909 alone). When administered alone, SB 206553 (10 mg/kg, i.p.) had no effect on adjunctive drinking (60.3 ± 3.8 ml).
In additional studies (in a separate group of animals), the effects of WAY-163909 were evaluated in the presence of either the selective 5-HT2C receptor antagonist SB 242084 (1 mg/kg, i.p.) or the selective 5-HT2B receptor antagonist SB 215505 (3 mg/kg, p.o.) to determine the relative contribution of 5-HT2C vs 5-HT2B receptors in mediating the effects of WAY-163909. In this group of rats, the effects of WAY-163909 were a little more potent than previously observed, with 3 mg/kg producing a marked effect on adjunctive drinking (−82%, p < 0.001). Pretreatment with the 5-HT2C antagonist SB 242084 (1 mg/kg, i.p.) blocked the decrease in adjunctive drinking produced by 3 mg/kg WAY-163909 (Fig. 4c; p < 0.001 vs 3 mg/kg WAY-163909 alone). When administered alone, SB 242084 (1 mg/kg, i.p.) had no effect on adjunctive drinking (55.4 ± 8.49 ml). In contrast, pretreatment with SB 215505 (3 mg/kg, p.o.) did not block the effects of WAY-163909.
Sexual dysfunction
In vehicle-treated rats, there were 2.4 ± 0.5 noncontact penile erections during the experimental session. Although the overall ANOVA did not reveal significant effects of WAY-163909 [F(2,23) = 2.32, p = 0.12], planned post hoc comparisons revealed that the high dose of WAY-163909 (10 mg/kg) caused a 53% decrease in the number of noncontact penile erections relative to vehicle-treated subjects to 1.1 ± 0.4 noncontact penile erections (p = 0.04; Fig. 5).
Discussion
WAY-163909 is a novel compound that binds with high affinity and functions as a full agonist at the 5-HT2C receptor (Dunlop et al. 2005). Consistent with its 5-HT2C agonist profile, WAY-163909 decreases food intake and body weight in mice and rats, effects that are antagonized by 5-HT2C, but not 5-HT2A or 5-HT2B antagonists (Dunlop et al. 2005). In addition, WAY-163909 is also effective in multiple animal models of antipsychotic-like activity (Marquis et al. 2007). The present studies were conducted to investigate effects of WAY-163909 in multiple animal models of antidepressant-like activity. These models were chosen based on their sensitivity to the effects of clinically effective antidepressants such as the SSRI fluoxetine or the NRI desipramine (Table 2).
WAY-163909 significantly decreased immobility in the FST in both SD and WKY rats. In the SD rats, WAY-163909 increased swimming and had no effect on climbing, a profile similar to that observed with SSRIs. The reductions in immobility produced by WAY-163909 in the WKY rats were fully reversed by the 5-HT2C/2B receptor antagonist SB 206553, indicative of a role for 5-HT2C and/or 5-HT2B receptors in mediating this behavioral effect. Previous studies have shown that structurally diverse 5-HT2C receptor agonists such as WAY-161503 or Ro 60-0175 also decrease immobility and increase swimming (Cyran et al. 2000). Comparatively high doses of WAY-163909 (10 or 17 mg/kg) were required to produce antidepressant-like effects in this model, which might suggest that activities of WAY-163909 at other receptors, such as 5-HT2A or 5-HT2B might play a role in mediating this effect. However, it is noteworthy that this is the dose range where anorectic effects of WAY-163909 are observed, effects that are fully antagonized by the 5-HT2C selective antagonist SB 242084 but not by 5-HT2A or 5-HT2B receptor antagonists (Dunlop et al. 2005).
The effects of acute treatment with WAY-163909 were also evaluated in the resident–intruder model. Acute treatment with a wide range of antidepressant drugs, including SSRIs, serotonin–noradrenaline reuptake inhibitors (SNRIs), and 5-HT2C receptor agonists, all reduce the aggressive behavior of the resident rat at doses that are not associated with motor impairment (Mitchell and Redfern 2005). Similarly, acute treatment with WAY-163909 selectively reduced the aggressive behavior of resident rats during social encounters with drug-free unfamiliar intruder conspecifics. In contrast, higher doses of WAY-163909 were required to similarly reduce the total behavior score of the resident rats (ID50 = 0.97 mg/kg). These observations indicate that acute treatment with WAY-163909 reduces aggressive behavior at doses that do not result in motor impairment. Interestingly, WAY-163909 failed to induce 5-HT2A receptor-mediated head/body shake behavior at any of the doses examined. This observation is consistent with the known selectivity of WAY-163909 for 5-HT2C receptors over 5-HT2A receptors and the lack of functional agonism in vitro and suggests that WAY-163909 may be devoid of agonist activity at 5-HT2A receptors in vivo.
Chronic, but not acute, administration of clinically effective antidepressants has been shown to be effective in the BULB model in rats. More specifically, 14 or 21-day administration of SSRIs or NRIs appears to be necessary to produce a decrease in the hyperactivity associated with BULB (Kelly et al. 1997; Redmond et al. 1997; Cryan et al. 1998, 1999; Harkin et al. 1999; Mar et al. 2000). However, 5-HT2C receptor agonists may have more rapid onset of activity based on their direct activation of the 5-HT2C receptor rather than the indirect activation that occurs over time produced by SSRIs. To this end, previous studies have reported that 5-HT2C receptor agonists show rapid onset antidepressant-like effects in the chronic mild stress model and the BULB model (Moreau et al. 1993, 1996; Martin et al. 1998). In the present study, WAY-163909 was administered for either 5 or 21 days to both sham and bulbectomized rats. WAY-163909 effectively decreased the hyperactivity associated with BULB without affecting levels of activity in sham operated rats after both short-term and long-term treatment, consistent with rapid onset antidepressant-like effects. Moreover, as tolerance develops to some, but not all effects of 5-HT2C receptor agonists (Freo et al. 1992; Kennedy et al. 1993; Rosenzweig-Lipson et al. 2006; Vickers et al. 2000, 2003; McCall et al. 2001; Hayashi et al. 2004), it is important to note that the effects observed at 5 days in the BULB model were still present at 21 days. The lack of tolerance is consistent with other effects of WAY-163909 such as those on food intake and body weight (Dunlop et al. 2005) and on the atypical antipsychotic-like neurochemical, electrophysiological, and behavioral profile that occurs after both acute and 21-day administration (Rosenzweig-Lipson et al. 2005).
WAY-163909 was evaluated in a novel model (Sukoff Rizzo et al. 2007) of antidepressant-induced sexual dysfunction. In this model, male rats that had a previous overnight sexual experience are placed in a room with receptive females that provide visual, auditory and olfactory cues. Under these conditions, there is an increase in the number of spontaneous penile erections. Antidepressants decrease the number of spontaneous penile erections. The rank order of the magnitude of the antidepressant-induced attenuation of spontaneous penile erection is comparable to the rank order of the magnitude of antidepressant-induced sexual dysfunction observed clinically (buproprion, minimal; desipramine, modest; fluoxetine, most severe). WAY-163909 produced decreases in sexual function in this model at 10 mg/kg. For comparison, chronic administration of WAY-163909 reverses BULB-induced hyperactivity at 3 mg/kg, indicating a threefold window of separation. In comparison, both fluoxetine and desipramine decrease sexual function at doses that fully reverse BULB-induced hyperactivity, indicative of no therapeutic window (Sukoff Rizzo et al. 2007).
Several lines of evidence suggest that 5-HT2C receptor agonists may be effective treatments for OCD. For example, 5-HT2C receptor agonists have been shown to be effective in animal models of compulsive behavior, such as SIP, 8-OH-DPAT-induced scratching in squirrel monkeys, marble burying, and excessive eating of palatable foods (Bos et al. 1997; Martin et al. 1998). Additionally, 5-HT2C knockout mice exhibit compulsive-like behaviors (Chou-Green et al. 2003). The present studies demonstrate that WAY-163909 produces dose-dependent decreases in adjunctive drinking. It is important to note that these effects are not simply a result of decreased appetitive behaviors (food or water intake). In the SIP condition, rats continued to eat all the pellets at the doses tested. Additionally, in the control condition, rats ate all the pellets, and normal water consumption was not significantly affected. Moreover, the 5-HT2C/2B receptor antagonist SB 206553 and the more selective 5-HT2C receptor antagonist SB 242084, but not the 5-HT2B receptor antagonist SB 2155505 blocked the effects of WAY-163909, consistent with the pharmacological mechanism of action of WAY-163909 being mediated via stimulation of the 5-HT2C receptor. These results are consistent with previous studies demonstrating that acute administration of 5-HT2C receptor agonists decrease SIP and contrast with the effects of SSRIs that typically require chronic administration in this model (Woods et al. 1993; Martin et al. 1998; Hogg and Dalvi 2004). It has been suggested that SIP may also be an appropriate model for detecting the onset of activity of antidepressant compounds insomuch as combining the activity of 5-HT1A or 5-HT1B antagonists with SSRIs results in an acute effect in this model (Hogg and Dalvi 2004). Taken together with previous studies demonstrating that 5-HT2C receptor agonists are effective in animal models of compulsive behaviors, these results suggest that WAY-163909 may be an effective treatment for OCD.
The antidepressant-like effects of 5-HT2C receptor agonists appear to conflict with data suggesting that 5-HT2C receptor antagonists, particularly in combination with SSRIs, result in elevated levels of 5-HT and augment the effects of SSRIs in the mouse tail suspension test (Cremers et al. 2004). The antagonist studies are supported by similar findings in 5-HT2C receptor knockout mice (Cremers et al. 2004). One might argue that the antidepressant-like effects of 5-HT2C agonists were merely a reflection of a down-regulated or desensitized 5-HT2C receptor. However, this seems unlikely as the behavioral effects of WAY-163909 across multiple behavioral, electrophysiological, and neurochemical assays are similar after acute and chronic administration (Dunlop et al. 2005; Rosenzweig-Lipson et al. 2005; Marquis et al. 2007). Moreover, the acute effects of WAY-163909 in multiple models can be antagonized by 5-HT2C receptor antagonists suggesting that it is unlikely that the acute effects reflect rapid desensitization (Dunlop et al. 2005; present studies). Interestingly, there are also studies demonstrating that the antidepressant-like effects of fluoxetine in the forced swim test can be reversed by 5-HT2C receptor antagonists (Cryan and Lucki 2000) and data suggesting that 5-HT2C receptor agonists augment the effects of SSRIs in the mouse forced swim test (Clenet et al. 2001). Despite this conflicting literature, it is possible that both hypotheses may be accurate. For example, if the combination of 5-HT2C receptor antagonists with SSRIs results in elevated 5-HT, which in turn acts at a 5-HT receptor other than the 5-HT2C receptor, such as the 5-HT1A receptor, it might be possible to see antidepressant-like effects via that alternative receptor.
The wide array of behavioral changes associated with 5-HT2C receptor agonism is quite interesting. In this regard, WAY-163909 produces anorectic effects, antipsychotic-like effects, and antidepressant-like effects (Dunlop et al. 2005; Marquis et al. 2007). The ability of 5-HT2C receptor antagonists to block the effects of WAY-163909 across these multiple domains is consistent with WAY-163909 producing its effects via activation of the 5-HT2C receptor. 5-HT2C receptors are widely expressed in brain and that may, in part, explain the diversity of roles for 5-HT2C receptors. As it has long been known that 5-HT has a wide diversity of functional activities, the widespread distribution of the 5-HT2C receptor, may allow activation of this receptor to mimic many of the effects of 5-HT.
Taken together, the results of the present studies clearly demonstrate the antidepressant-like effects of the novel selective 5-HT2C receptor agonist WAY-163909 in multiple models of antidepressant activity. These results are consistent with the known literature effects of other less selective 5-HT2C agonists in these and other antidepressant models. Insomuch as faster onset and sexual dysfunction are two key areas for differentiating future antidepressants (Rosenzweig-Lipson et al. 2007), it is possible that 5-HT2C receptor agonists, and more specifically WAY-163909, may be an effective antidepressant and may have more rapid onset than current pharmacotherapies for either OCD or depression without concomitant sexual side effects.
Abbreviations
- SSRI:
-
serotonin reuptake inhibitor
- BULB:
-
olfactory bulbectomy
- 5-HT:
-
serotonin
- FST:
-
forced swim test
- (7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]-diazepino[6,7,1hi]indole:
-
WAY-163909
- ANOVA:
-
analysis of variance
- SEM:
-
standard error of the mean
- DRL:
-
differential reinforcement of low rate
- LSD:
-
least significant difference
- OCD:
-
obsessive–compulsive disorder
- SIP:
-
schedule-induced polydipsia
- SD:
-
Sprague-Dawley
- WKY:
-
Wistar-Kyoto
References
Beyer CE, Boikess S, Luo B, Dawson LA (2002) Comparison of the effects of antidepressants on norepinephrine and serotonin concentrations in the rat frontal cortex: an in-vivo microdialysis study. J Psychopharmacol 16:297–304
Bickerdike MJ, Adams DR, Bentley J, Benwell KR, Cliffe IA, Kennett GA, Knight AR, Malcolm CS, Misra A, Quirk K, Roffey JRA, Dourish CT (2002) Radioligand binding profile and in vitro functional efficacy of VER-3323, a novel 5-HT2C/5-HT2B receptor agonist. 5th IUPHAR Satellite Meeting on Serotonin
Bos M, Jenck F, Martin JR, Moreau JL, Sleight AJ, Wichmann J, Widmer U (1997) Novel agonists of 5HT2C receptors. Synthesis and biological evaluation of substituted 2-(indol-1-yl)-1-methylethylamines and 2-(indeno[1,2-b]pyrrol-1-yl)-1-methylethylamines. Improved therapeutics for obsessive compulsive disorder. J Med Chem 15:2762–2769
Chou-Green JM, Holscher TD, Dallman MF, Akana SF (2003) Compulsive behavior in the 5-HT2C receptor knockout mouse. Physiol Behav 78:641–649
Clenet F, De Vos A, Bourin M (2001) Involvement of 5-HT2C receptors in the anti-immobility effects of antidepressants in the forced swimming test in mice. Eur Neuropsychopharmacol 11:145–152
Cremers TI, Giorgetti M, Bosker FJ, Hogg S, Arnt J, Mork A, Honig G, Bogeso K-P, Westerink B, den Boer BHC, Wikstrom HV, Tecott LH (2004) Inactivation of 5-HT(2C) receptors potentiates consequences of serotonin reuptake blockade. Neuropsychopharmacology 29:1782–1789
Cryan JF, Lucki I (2000) Antidepressant-like behavioral effects mediated by 5-hydroxytryptamine2C receptors. J Pharmacol Exp Ther 295:1120–1126
Cryan JF, McGrath C, Leonard BE, Norman TR (1998) Combining pindolol and paroxetine in an animal model of chronic antidepressant action—can early onset of action be detected? Eur J Pharmacol 352:23–28
Cryan JF, McGrath C, Leonard BE, Norman TR (1999) Onset of the effects of the 5-HT1A antagonist, WAY-100635, alone, and in combination with paroxetine, on olfactory bulbectomy and 8-OH-DPAT-induced changes in the rat. Pharmacol Biochem Behav 63:333–338
Dawson LA, Nguyen HQ, Smith DI, Schechter LE (2000) Effects of chronic fluoxetine treatment in the presence and absence of (±)pindolol: a microdialysis study. Br J Pharmacol 130:797–804
Detke MJ, Rickels M, Lucki I (1995) Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology 119:47–54
Dunlop J, Sabb AL, Mazandarani H, Zhang J, Kalgaonker S, Shukhina E, Sukoff S, Vogel R, Stack G, Schechter L, Harrison BL, Rosenzweig-Lipson S (2005) WAY-163909 (7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]-diazepino[6,7,1hi]indole); A novel 5-HT2C receptor selective agonist with anorectic activity. J Pharmacol Exp Ther 313(2):862–869
Dunlop J, Marquis M, Kim HL, Leung L, Kao J, Cheesman C, Rosenzweig-Lipson S (2006) Pharmacological profile of the 5-HT2C receptor agonist WAY-163909 ((7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole); therapeutic potential in multiple indications. CNS Drug Review 12:167–176
Freo U, Holloway HW, Greig NH, Soncrant TT (1992) Chronic treatment with meta-chlorophenylpiperazine (m-CPP) alters behavioral and cerebral metabolic responses to the serotonin agonists m-CPP and quipazine but not 8-hydroxy-2(di-N-propylamino)tetralin. Psychopharmacology 107:30–38
Harkin A, Kelly JP, McNamara M, Connor TJ, Dredge K, Redmond A, Leonard BE (1999) Activity and onset of action of reboxetine and effect of combination with sertraline in an animal model of depression. Eur J Pharmacol 364:123–132
Hayashi A, Sonoda R, Kimura Y, Takasu T, Suzuki M, Sasamata M, Miyata K (2004) Antiobesity effect of YM348, a novel 5-HT2C receptor agonist in Zucker rats. Brain Res 1011:221–227
Hogg S, Dalvi A (2004) Acceleration of onset of action in schedule-induced polydipsia: combinations of SSRI and 5-HT1A and 5-HT1B receptor antagonists. Pharmacol Biochem Behav 77:69–75
Kelly JP, Wrynn AS, Leonard BE (1997) The olfactory bulbectomized rat as a model of depression: an update. Pharmacol Ther 74:299–316
Kennedy AJ, Gibson EL, O’Connell MT, Curzon G (1993) Effects of housing, restraint and chronic treatments with mCPP and sertraline on behavioural responses to mCPP. Psychopharmacology 113:262–268
Kennett GA, Wood, MD, Bright F, Trail B, Riley G, Holland V, Avenell KY, Stean T, Upton N, Bromidge S, Forbes IT, Brown AM, Middlemiss DN, Blackburn TP (1997) SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology 36:609–620
Kennett GA, Trail B, Bright F (1998) Anxiolytic-like actions of BW 723C86 in the rat Vogel conflict test are 5-HT2B mediated. Neuropharmacology 36:609–620
Kimura Y, Hatanaka K, Naitou Y, Maeno K, Shimada I, Koakutsu A, Wanibuchi F, Yamaguchi T (2004) Pharmacological profile of YM348, a novel, potent and orally active 5-HT2C receptor agonist. Eur J Pharmacol 283:37–43
Leyson D, Kelder J (1998) Ligands for the 5-HT2C receptor as potential antidepressants and anxiolytics. In: van der Groot J (ed) Trends in drug research. Elsevier, Amsterdam, pp 49–61
Mar A, Spreekmeester E, Rochford J (2000) Antidepressants preferentially enhance habituation to novelty in the olfactory bulbectomized rat. Psychopharmacology 150:52–60
Marquis K, Sabb A, Logue SF, Stack G, Brennan JA, Piesla MJ, Comery TA, Grauer SM, Ashby CR, Nguyen HQ, Dawson LA, Stack G, Meltzer HY, Harrison BL, Rosenzweig-Lipson S (2007) WAY-163909 ((7bR,10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole): a novel 5-HT2C receptor selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther 320:486–496
Martin JR, Bos M, Jenck F, Moreau J, Mutel V, Sleight AJ, Wichmann J, Andrews JS, Berendsen HH, Broekkamp CL, Ruigt GS, Kohler C, Delft AM (1998) 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther 286:913–924
McCall RB, Franklin SR, Hyslop DK, Knauer CS, Chio CL, Haber CL, Fitzgerald LW (2001) PNU-22394, a 5-HT2C receptor agonist, reduces feeding in rodents and produces weight loss in humans. Soc Neurosci Abstr 27:309.2
Mitchell PJ, Redfern PH (1992) Acute and chronic antidepressant drug treatments induce opposite effects in the social behaviour of rats. J Psychopharmacol 6:241–257
Mitchell PJ, Redfern PH (2000) Effects of m-chlorophenylpiperazine and mesulergine on rodent agonistic behaviour. J Psychopharmacol 14:A32 (PD2)
Mitchell PJ, Redfern PH (2005) Animal models of depressive illness: the importance of chronic drug treatment. Curr Pharm Des 11:171–203
Moreau JL, Jenck F, Martin JR, Perrin S, Haefely WE (1993) Effects of repeated mild stress and two antidepressant treatments on the behavioral response to 5HT1C receptor activation in rats. Psychopharmacology 110:140–144
Moreau J-L, Bos M, Jenck F, Martin JR, Mortas P, Wichmann J (1996) 5HT2C receptor agonists exhibit antidepressant-like properties in the anhedonia model of depression in rats. Eur Neuropharmacol 6:169–175
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Redmond AM, McNamara MG, Dredge K, Kelly JP, Leondard BE (1997) Onset of action of venlafaxine, citalopram, and desipramine in the OB rat model of depression. J Psychopharmacol 11:A40
Rosenzweig-Lipson S, Beyer C, Malberg J, Lin Q, Ashby CR, Graf R, Sung A, Grauer S, Logue S (2005) WAY-163909, a 5-HT2C selective agonist for the treatment of obesity, depression and schizophrenia: lack of tolerance. SFN Itinerary Viewer
Rosenzweig-Lipson S, Zhang J, Mazandarani H, Harrison BL, Sabb A, Sabalski J, Stack G, Welmaker G, Barrett JE, Dunlop J (2006) Antiobesity-like effects of the 5-HT2C receptor agonist WAY-161503. Brain Res 1073–1074:240–251
Rosenzweig-Lipson S, Beyer CE, Hughes ZA, Khawaja X, Rajarao SJ, Malberg JE, Rahman Z, Ring RH, Schechter LE (2007) Differentiating antidepressants of the future: efficacy and safety. Pharmacol Ther 113:134–153
Sabb AL, Vogel RL, Welmaker G, Sabalski JE, Coupet J, Dunlop J, Rosenzweig-Lipson S, Harrison B (2004) Cycyloalkyl[b][1,4]benzodiazepinoindoles are agonists at the human 5-HT2C receptor. Bioorg Med Chem Lett 14:2603–2607
Sukoff Rizzo SJ, Schechter LE, Rosenzweig-Lipson S (2007) A novel approach for predicting antidepressant-induced sexual dysfunction in rats. Psychopharmacology, in press
Vickers SP, Benwell KR, Porter RH, Bickerdike MJ, Kennett GA, Dourish CT (2000) Comparative effects of continuous infusion of mCPP, Ro 60–0175 and d-fenfluramine on food intake, water intake, body weight and locomotor activity in rats. Br J Pharmacol 130:1305–1314
Vickers SP, Easton N, Webster LJ, Wyatt A, Bickerdike MJ, Dourish CT, Kennett GA (2003) Oral administration of the 5-HT2C receptor agonist, mCPP, reduces body weight gain in rats over 28 days as a result of maintained hypophagia. Psychopharmacology 167:274–280
Welmaker GS, Nelson JA, Sabalski JE, Sabb AL, Potoski JR, Graziano D, Kagan K, Coupet J, Dunlop J, Mazandarani H, Rosenzweig-Lipson S, Sukoff S, Zhang Y (2000) Synthesis and 5-Hydroxytryptamine (5-HT) activity of 2,3,4,4a-tetrahydro-1H-pyrazino[1,2-a]quinoxalin-5-(6H)ones and 2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,2-a]quinoxalines. Bioorg Med Chem Lett 10:1991–1994
Woods A, Smith C, Szewczak M, Dunn RW, Cornfeldt M, Corbett R (1993) Selective serotonin reuptake inhibitors decrease schedule-induced polydipsia in rats: a potential model for obsessive–compulsive disorder. Psychopharmacology 112:195–198
Acknowledgements
The authors would like to thank Menelas Pangalos and Ron Magolda for their unwavering support of 5-HT2C agonist studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenzweig-Lipson, S., Sabb, A., Stack, G. et al. Antidepressant-like effects of the novel, selective, 5-HT2C receptor agonist WAY-163909 in rodents. Psychopharmacology 192, 159–170 (2007). https://doi.org/10.1007/s00213-007-0710-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0710-6