Abstract
Rationale
Sex differences have been reported for the impact of nicotine and nonpharmacological cues on smoking. While nonpharmacological environmental stimuli have also been shown to influence nicotine self-administration in rats, there have been no attempts to examine the impact of sex differences in the contributions of nicotine and nondrug stimuli to this behavior.
Objectives
This experiment investigated sex differences in operant responding for nicotine in rats when drug infusions were delivered either in the absence of, or in combination with, a nonpharmacological stimulus.
Methods
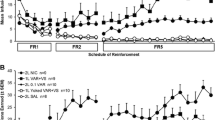
Initially, male and female rats acquired self-administration for nicotine alone across a range of doses (0.03, 0.06, and 0.15 mg kg−1 inf−1, freebase). After stable acquisition, nicotine infusions were combined with a weakly reinforcing, compound visual stimulus.
Results
While there was no overall effect of dose on active lever responding for nicotine in the absence of the visual stimulus, female rats responded more on the reinforced lever than males at 0.06 and 0.15 mg kg−1 inf−1 on an FR5 schedule. However, they also showed increased responding on the nonreinforced lever compared to males at the same doses. Combining nicotine infusions with the visual stimulus doubled responding compared to nicotine alone at 0.03 and 0.06, but not at 0.15 mg kg−1 inf−1: this effect was significantly greater for female rats.
Conclusions
These data highlight the prominent contribution of nonpharmacological stimuli to nicotine-reinforced behavior across a range of doses in both male and female rats. They also reveal sex differences in operant responding for nicotine under conditions where a nonpharmacological stimulus is either absent, or combined with drug delivery.




Similar content being viewed by others
References
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2001) Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav 70:515–530
Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF (2002) Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology 163:230–237
Campbell UC, Morgan AD, Carroll ME (2002) Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Depend 66(1):61–69
Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen S, Sved AF (2003) Enhancement of reinforced operant responding by nicotine and cocaine: analysis of dose and drug-contingency. Abstr Soc Neurosci, Prog. 323.8
Corrigall WA, Coen KM (1989) Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology 99:473–478
Corrigall WA, Coen KM, Adamson KL (1994) Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653(1–2):278–284
Cosgrove KP, Carroll ME (2003) Effects of a non-drug reinforcer, saccharin, on oral self-administration of phencyclidine in male and female rhesus monkeys. Psychopharmacology 170(1):9–16
Donny E, Caggiula AR, Knopf S, Brown C (1995) Nicotine self-administration in rats. Psychopharmacology 122:390–394
Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF (1998) Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology 136:83–90
Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE (1999) Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 147:135–142
Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S (2000) Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology 151:392–405
Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF (2003) Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology 169(1):68–76
Due DL, Huettel SA, Hall WG, Rubin DC (2002) Activation of mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am J Psychiatry 159:954–960
Everitt BJ, Wolf ME (2002) Psychomotor stimulant addiction: a neural systems perspective. J Neurosci 22(9):3312–3320
Goldberg SR, Spealman RD, Risner ME, Henningfield JE (1983) Control of behavior by intravenous nicotine injections in laboratory animals. Pharmacol Biochem Behav 19(6):1011–1020
Hu M, Crombag HS, Robinson TE, Becker JB (2004) Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29(1):81–85
Koylu E, Demirgoren S, London ED, Pogun S (1997) Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci 61(12):PL185–PL190
Kruzich PJ, Congleton KM, See RE (2001) Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behav Neurosci 115(5):1086–1092
Kyerematen GA, Owens GF, Chattopadhyay B, deBethizy JD, Vesell ES (1988) Sexual dimorphism of nicotine metabolism and ditribution in the rat. Studies in vivo and in vitro. Drug Metab Dispos 16:823–828
Lanza ST, Donny EC, Collins LM, Balster RL (2004) Analyzing the acquisition of drug self-administration using growth curves. Drug Alcohol Depend 75(1):11–21
Lynch WJ, Carroll ME (2000) Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology 148(2):196–200
Olausson P, Jentsch JD, Taylor JR (2004a) Nicotine enhances responding with conditioned reinforcement. Psychopharmacology 171(2):173–178
Olausson P, Jentsch JD, Taylor JR (2004b) Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology 173(1–2):98–104
Perkins KA (1999) Nicotine discrimination in men and women. Pharmacol Biochem Behav 64(2):295–299
Perkins KA, Epstein LH, Grobe JE, Fonte C (1994) Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol Biochem Behav 47:107–112
Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S (2001) Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res 3(2):141–150
Perkins KA, Jacobs L, Sanders M, Caggiula AR (2002) Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology 163(2):194–201
Phillips AG, Fibiger HC (1990) Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behav Pharmacol 1:228–269
Pogun S (2001) Sex differences in brain and behavior: emphasis on nicotine, nitric oxide and place learning. Int J Psychophysiol 42(2):195–208
Robbins TW, Koob GF (1978) Pipradrol enhances reinforcing properties of stimuli paired with brain stimulation. Pharmacol Biochem Behav 8:219–222
Robbins TW, Watson BA, Gaskin M, Ennis C (1983) Contrasting interactions of pipradrol, d-amphetamine, cocaine, cocaine analogues, apomorphine and other drugs with conditioned reinforcement. Psychopharmacology 80:113–119
Schepers G, Rustemeier K, Walk RA, Hackenberg U (1993) Metabolism of S-nicotine in noninduced and aroclor-induced rats. Eur J Drug Metab Pharmacokinet 18(2):187–197
See RE (2002) Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 71(3):517–529
Taylor JR, Horger BA (1999) Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology 142(1):31–40
USDHHS (1988) Nicotine addiction: a report of the surgeon general. US Department of Health and Human Services, Office of the Assistant Secretary for Health, Office on Smoking and Health, Rockville, MD
Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA (2003) Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci 23(8):3176–3185
Woolverton WL, Nader MA (1990) Experimental evaluation of the reinforcing effects of drugs. In: Adler MW, Cowan A (eds) Testing and evaluation of drugs of abuse, modern methods in pharmacology, vol 6. Wiley-Liss, New York, pp 165–192
Acknowledgements
This work was supported by National Institute on Drug Abuse research grants, DA-10464 and DA-12655, and by a Howard Hughes Predoctoral Research Fellowship awarded to N. Chaudhri. “Principles of laboratory animal care” (NIH No. 85-23, revised 1985) were followed throughout all experiments. The University of Pittsburgh Institutional Animal Care and Use Committee, Assurance Number A3187-01 approved this research. N. Chaudhri can be contacted at nchaudhri@egcrc.net.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaudhri, N., Caggiula, A.R., Donny, E.C. et al. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology 180, 258–266 (2005). https://doi.org/10.1007/s00213-005-2152-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-2152-3